Difference between revisions of "Laboratory Techniques"
| Line 722: | Line 722: | ||
===External Resources=== | ===External Resources=== | ||
| + | * [https://www.youtube.com/watch?v=5SWOFBhdvrY ASBC Webinar: Green Chemistry & Beer Science by Dana Garves of Oregon BrewLab.] | ||
* [https://journals.asm.org/doi/pdf/10.1128/jmbe.00336-21 Bootleg Biology: a Semester-Long CURE Using Wild Yeast to Brew Beer.] | * [https://journals.asm.org/doi/pdf/10.1128/jmbe.00336-21 Bootleg Biology: a Semester-Long CURE Using Wild Yeast to Brew Beer.] | ||
* [https://www.brewerspublications.com/products/yeast-the-practical-guide-to-beer-fermentation "Yeast: The Practical Guide to Beer Fermentation," by: Chris White and Jamil Zainasheff.] | * [https://www.brewerspublications.com/products/yeast-the-practical-guide-to-beer-fermentation "Yeast: The Practical Guide to Beer Fermentation," by: Chris White and Jamil Zainasheff.] | ||
Revision as of 18:42, 13 June 2024
This focuses on home lab and small brewery lab techniques.
Contents
- 1 Equipment
- 2 Growth Media
- 3 Storage
- 4 Propagators
- 5 Techniques
- 5.1 Aseptic Technique
- 5.2 Making Agar Plates
- 5.3 Yeast/Bacteria Isolation
- 5.4 Making Your Own Media
- 5.5 Yeast Banking
- 5.6 Cell Counting
- 5.7 Gram Staining
- 5.8 Propagating Yeast
- 5.9 Semi-Anaerobic Containers for Incubating Plates
- 5.10 Identification
- 5.11 Yeast Rinsing/Washing
- 5.12 Hybridization and Modification
- 6 Shipping Cultures
- 7 Laboratory Information Management Systems
- 8 Chemistry
- 9 Historical
- 10 See Also
- 11 References
Equipment
General (links to other pages)
- Nonconventional Yeasts and Bacteria
- Quality Assurance
- Microscope
- How To Use a Microscope
- pH Meter
- MTF thread on advice for what kind of pipette to get for cell counting.
- Teri Fahrendorf’s "Small Brewery Lab Procedures Manual"
- Colorado Brewers Guild "So You Want to Add a Brewing Lab?"
Bunsen Burner
Density Meter
Density Meter's are a much more accurate tool for testing things like ethanol content as well as other things that can be useful in a brewery setting.
Dissolved Oxygen Meter
Dissolved oxygen meters play a large role in brewery QC of the final packaged products as well as oxygenation of wort pre-fermentation. Although cheaper DO meters can be used to find the PPM(parts per million) of oxygen in the wort pre-fermentation, more expensive advanced equipment is needed post fermentation as you'll need equipment that is capable of reading much lower levels of DO on a ppb level. When monitoring DO its crucial to be under 100 ppb packaged DO and for hop driven beers its ideal to be under 50 ppb or else you risk oxidation and other off flavors associated with oxygen.
During DO packaging testing, Justin Amaral found that breweries have issues with DO coming directly from their canning/bottling system. The most common factor found in high DO readings was in proper purging of lines when starting up the machines or taking a break as well as mechanical failure.
Orbital Shaker
An orbital shaker is a laboratory device used for mixing substances or maintaining movement of fluids. Maintaining movement of liquids has been shown to help some microorganisms grow. For example, running a shaker at 80 RPM for Brettanomyces starters is an effective way to grow this genra (see Brettanomyces starters) [1]. For a home orbital shaker example, see Example of a Home Lab Orbital Shaker.
PCR/qPCR
https://www.minipcr.com/product/minipcr-dna-discovery-system/
https://www.facebook.com/groups/MilkTheFunk/permalink/1888017211226484/ - Under Joe I's comment
https://www.weberscientific.com/beer-spoilage-micro-test-kit-microbiologique (see this MTF thread).
https://www.facebook.com/groups/MilkTheFunk/permalink/2069735766387960/
"PCR Basics For Brewers", presentation by Escarpment Labs.
Diastatic strains of S. cerevisiae
- MTF thread on identifying diastatic strains of Saccharomyces cerevisiae using "Instagene". Richard Preiss warns of false positives when using STA1 PCR.
- See also: diastatic strains of S. cerevisiae.
Titratable Acidity Meter
TA meters can be a very useful tool in sour beer brewing. Although its generally been used in the wine world to measure the acidity of wines, it can be used on beer as well. Although pH can give you rough look how acidic something is, it doesn't really give you a accurate outlook on how acidic something is on the palate. Please refer to http://www.milkthefunk.com/wiki/Titratable_Acidity for more info.
UV/VIS Spectrophotometer
A Spectrophotometer can be used for an array of tests. They are commonly used to analyze SRM, IBU, ethanol content and other wort compositions. They can also be used for cell counts as well as other microbiology facets.
UV Plate Cooling Cabinet
Using a UV sterilization cabinet can not only help cool/sterilize freshly poured plates but it can be used to UV sterilize other things as well. The build itself is quite simple. You will have to do some basic wiring.
Ideally you'll want a Laminar flow hood with a UV light in the hood, but this will do for just cooling plates or doing quick UV sterilization.
Parts List -
Light Ballast https://www.amazon.com/gp/product/B00AB32J7S/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 Lamp Mount https://www.amazon.com/gp/product/B0036ZA966/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 4 pin connector https://www.amazon.com/gp/product/B003B92NP2/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 UV Light https://www.amazon.com/gp/product/B001HB3E2W/ref=oh_aui_detailpage_o06_s00?ie=UTF8&psc=1 Plexiglass https://www.amazon.com/gp/product/B019D0DUDQ/ref=oh_aui_detailpage_o03_s00?ie=UTF8&psc=1
Growth Media
General Notes
- MRS media and some other media types are not food grade, and should only be used for initial propagation from frozen stocks (and afterwards decanted or pelleted) or for making agar for isolation. MRS media should not be used for large propagation such as starters or yeast propagators at breweries [2].
- Richard Preiss from Escarpment Laboratories reported better agar results when limiting autoclave times to 15 minutes for recipes that include copper-containing ingredients [3].
- Cycloheximide will inhibit Saccharomyces and Brettanomyces at 100µg/ml [4].
See also:
- MTF tips on autoclaving wort extract based media in such a way that prevents precipitation and darkening.
- MTF tips on what media to use for wild yeast isolation.
- For techniques on separating yeast strains from themselves and from bacteria, see Yeast/Bacteria Isolation.
- To detect Brettanomyces in a Saccharomyces culture, WLN (or WLN along with DBDM) or even something as cheap as YPD can be used because Saccharomyces will show significant colony growth at 3 days, while Brettanomyces colonies will grow at 5-10 days [5].
Reference on DBDM: https://www.facebook.com/groups/MilkTheFunk/permalink/1805210829507123/?comment_id=1805397166155156&comment_tracking=%7B%22tn%22%3A%22R%22%7D
Lactobacillus/Pediococcus
See Rogosa SL Agar.
MRS Media
| Chemical | Usage Amount |
|---|---|
| Dextrose | 20 grams |
| Peptone | 10 grams |
| Beef Extract | 8 grams |
| Yeast Extract | 4 grams |
| Sodium Acetate | 5 grams |
| Dipotassium Hydrogen Phosphate | 2 grams |
| Ammonium Citrate | 2 grams |
| Manganous Sulfate Tetrahydrate | 0.05 grams |
| Magnesium Sulfate Heptahydrate | 0.2 grams |
| Distilled/De-ionized Water | Fill to 1000 ML |
Catalase enzyme can be spread onto MRS media to assist with culturing so-called "viable but non-culturable" (VBNC) bacteria cells; see the Quality Assurance page for details.
NBB Media
NBB media can also be used to help detect/isolate spoilage bacteria. This is a pre-made media by Doehler. More info can be found at NBB Media. Although ASBC suggests this media its most likely favored by them as their recommendations are generally products sold by Siebel. After a quick discussion with Richard Preiss cheaper media can be used to reach the same goals. Using HLP, WLN w/ cycloheximide and tween 80, and MRS w/ cycloheximide and tween 80 you can achieve the same results as using the array of NBB media available.
ABD Media
Advanced beer-spoiler detection medium (ABD), which is basically MRS with some of the MRS substituted for beer, has reportedly been shown to be a better growth medium for beer-spoiler LAB which have adapted to the brewing environment and are difficult to grow on other media. This media also has the advantage of inhibiting the growth of other microorganisms that are not beer spoilers. In order to grow some very slow growing strains, microcolony methods using carboxyfluorescein diacetate (CFDA) and species-specific fluorescence in situ hybridization (FISH) allows detection of slow-growing strains of LAB within 3 days, although the CFDA and FISH approaches require special equipment that might not be available for some QC laboratories [6][7].
| Chemical | Usage Amount |
|---|---|
| MRS broth (powder) | 2.61 grams |
| Sodium acetate | 0.5 grams |
| Clycloheximide | 10 milligrams |
| Agar | 15 grams |
| Beer (presumably lager beer with low IBU) | 1000 milliliters |
| Final pH | 5.0 |
The Yeast Bay Media
Nick Impellitteri from The Yeast Bay shared his formulation for a 1 liter formulation [8].
| Chemical | Usage Amount |
|---|---|
| Dextrose | 25 grams |
| Fermaid O nutrient | 10 grams |
| CaCO3 | 2.5 grams |
| Apple Juice (no preservatives) | 100 milliliters |
| Tomato Juice | 20 milliliters |
| Tween 80 | 1 milliliter |
| Distilled Water | 1 liter |
| Final pH | 6.2-6.3 |
HLP Media
Developed by the ASBC and BA. Hsu’s Lactobacillus and Pediococcus (HLP) media can be employed to screen for common beer spoilers, while selecting against other organisms, such as yeast, gram negative bacteria and aerobic microbes. This video outlines the ASBC Methods of Analysis technique for utilizing HLP media in a brewery. The benefit of this media is that it doesn't need to be autoclaved or incubated anaerobically, so it is a good option for small breweries with limited lab equipment.
See also: ASBC Methods of Analysis Training Videos and MBAA Podcast episode with Eric Jorgenson.
Misc
See also Lactobacillus starters for food safe, brewery-friendly formulations.
Saccharomyces
A wide variety of media can be used for Saccharomyces. Bromocresol Green can also be added to these media, as in the commercial WLN formulation. Most Saccharomyces cannot metabolize this dye, causing the colonies to stain green. Chloramphenicol can also be added to eliminate bacterial growth [9]. Although not all of these media are specifically for your average brewers Saccharomyces, most strains should have no issues growing.
YPD Media
| Chemical | Usage Amount |
|---|---|
| Yeast Extract | 10 grams |
| Peptone | 20 grams |
| Dextrose | 20 grams |
| Agar(optional) | 15 grams |
| Distilled Water | Fill to 1000 ML |
MYPG Media
| Chemical | Usage Amount |
|---|---|
| Malt Extract | 3 grams |
| Yeast Extract | 3 grams |
| Peptone | 3 grams |
| Dextrose | 10 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Sabouraud Media
| Chemical | Usage Amount |
|---|---|
| Cycloheximide (Optional) | 10 mg |
| Chloramphenicol (Optional) | 0.5 grams |
| Peptone | 5 grams |
| Dextrose | 20 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Freezing Media
| Chemical | Usage Amount |
|---|---|
| Glycerin | 50 grams |
| Ascorbic Acid | 15 grams |
| Liquid YPD/MYPG | Fill to 100 ML |
Beef Broth Media
| Chemical | Usage Amount |
|---|---|
| Beef Broth(No preservatives) | 500 mL |
| NaCI (can substitute non-iodized or sea salt) | 50-200 grams |
| Peptone | 5 grams |
| Dextrose | 10 grams |
| Agar(optional) | 17 grams |
| Distilled Water | Fill to 1000 ML |
Wild Yeast Screening Media
| Chemical | Usage Amount |
|---|---|
| Peptone | 5 grams |
| Yeast Extract | 3 grams |
| Malt Extract | 3 grams |
| Dextrose | 5 grams |
| CuSO4 | 310 mg |
| Distilled Water | Fill to 1000 ML |
Wild Yeast Medium from Sylvester et al. 2015
| Ammonium sulfate | 5 grams |
| Synthetic Complete Dropout mix (US Biological) | 1 gram |
| Yeast Nitrogen Base without amino acids, Carbohydrate & w/o AS (US Biological) | 1.72 grams |
| Ampicillin | 0.1 grams |
| Chloramphenicol | 0.03 grams |
| Glucose | 8% or 0.8% |
LCYM/LCSM Media (for wild yeast and diastatic strains of S. cerevisiae) [10]
| Chemical | Usage Amount |
|---|---|
| Ammonium chloride | 0.5 grams |
| Dipotassium ortho phosphate | 1.1 grams |
| Cupric sulphate (anhydrous) | 0.55 grams |
| Dextrose | 10 grams |
| DME | 2 grams |
| Peptone | 2 grams |
| Yeast Extract | 4 grams |
| Agar | 20 grams |
| Distilled/De-ionized Water | Fill to 1000 mL |
Omega-optimized LCYM Media (Autoclaved for no longer than 15 minutes to preserve copper-containing ingredients. This media is specifically for detecting diastatic strains of S. cerevisiae; reported more reliable detection than regular LCSM and Weber diastaticus media; see this MTF thread by Laura Burns from Omega Yeast Labs and the associated write up)
| Chemical | Usage Amount |
|---|---|
| Ammonium sulfate | 0.5 grams |
| Dipotassium ortho phosphate | 1 gram |
| Cupric sulphate (anhydrous) | 0.6 grams |
| Dextrose | 10 grams |
| DME | 2 grams |
| Peptone | 2 grams |
| Yeast Extract | 4 grams |
| Agar | 20 grams |
| Distilled/De-ionized Water | Fill to 1000 mL |
Escarpment CSSM (A modified version of the Omega LCYM Media. This media is specifically for detecting diastatic strains of S. cerevisiae; Also reported to culture beer strains of Brettanomyces, Pichia, and other wild yeasts[11] - see this MBAA paper.)
| Chemical | Usage Amount |
|---|---|
| Ammonium sulfate | 0.5 grams |
| potassium phosphate dibasic | 0.5 gram |
| Cupric sulphate (anhydrous) | 0.6 grams |
| Maltose | 0.5 grams |
| potato starch (Alfa Aesar no.213400) | 20 grams |
| Peptone | 2 grams |
| Liquid malt extract (Briess Ultralight) | 2 grams |
| Yeast extract | 4 grams |
| Agar | 20 grams |
| Distilled/De-ionized Water | Fill to 1000 mL |
Brettanomyces
A few different media can be used to isolate Brettanomyces but DBDM medium is commonly used. WLD with additions of cycloheximide can also be used. A simple Malt agar with cycloheximide has been shown to grow Brettanomyces that has been adapted to the brewing environment more efficiently than Dekkera medium (recommended by the European Brewing Convention, but not listed here) and universal beer medium (recommended by the Brewery Convention of Japan; also not listed here) [12]. DBDM may not allow all strains of Brettanomyces to grow [13]. The Escarpment CSSM media for diastatic S. cerevisiae is reported to also be a good growth medium for beer strains of Brettanomyces (see Saccharomyces above).
Renouf et al. (2007) and Comitini et al. (2019) demonstrated that an "enrichment Brettanomyces bruxellensis" media called EBB is more efficient at first growing up Brettanomyces before trying to culture it on DBDM. Brettanomyces was allowed to grow for 80 days in the EBB media, and then streaked onto DBDM for selection for Brettanomyces (other wild yeast such as Hanseniaspora and Pichia grew much more readily than Brettanomyces that was cultured from wine grapes) [14][15].
Due to the slow growth of Brettanomyces on traditional growth media, a new technique called indirect impedance, which utilizes electrical currents to measure the impedance of the electricity caused by microorganisms, has been proposed as a way to detect Brettanomyces in wine [16].
See also: Wild Isolation of Brettanomyces.
YPD
See this article by Dr. Bryan Heit on using a modified YPD media that is cheaper and easier to prepare than WLN, along with techniques to help improve the chances of culturing wild Brettanomyces while mostly eliminating other yeasts and mold.
| Chemical | Usage Amount |
|---|---|
| Yeast Extract | 10 grams |
| Peptone | 20 grams |
| Dextrose | 20 grams |
| Agar(optional) | 15 grams |
| Distilled Water | Fill to 1000 ML |
EBB Media Recipe
| Chemical | Usage Amount |
|---|---|
| Red grape juice (possibly replaceable with DME?) | 200 mL/L |
| Ethanol | 40 mL/L |
| Malt extract | 1.5 g/L |
| Yeast extract | 1.5 g/L |
| (NH4)2 SO4 (sourced from Sigma-Aldrich) | 0.5 g/L |
| MgSO4 | 0.2 g/L |
| Tween 80 | 0.5 mL/L |
| Biphenyl (limits mold) | 0.2 g/L (w/v) |
| Chloramphenicol (limits bacteria) | 0.05 g/L (w/v) |
| Adjust pH to 5.0 with sodium hydroxide |
Notes: gentle agitation during growth (120 rpm).
DBDM Media Recipe
| Chemical | Usage Amount |
|---|---|
| Yeast nitrogen base (YNB) | 6.5 grams |
| Ethanol | 4% v/v |
| Cycloheximide | 10 mg |
| p-coumaric acid | 100 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 mL |
WLD Media Recipe
| Chemical | Usage Amount |
|---|---|
| Cycloheximide | 4 grams |
| Yeast Extract | 4 grams |
| Pancreatic Digest of Casein(Peptone) | 5 grams |
| Dextrose | 50 grams |
| Monopotassium Phosphate | .55 grams |
| Potassium Chloride | 425 mg |
| Calcium Chloride | 125 mg |
| Magnesium Sulfate | 125 mg |
| Ferric Chloride | 2.5 mg |
| Manganese Sulfate | 2.5 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 mL |
Malt Agar Media Recipe
Click here for directions on how to make this medium. The below recipe is using the Suzuki (2008) recipe instead of the recipe in the linked directions.
| Malt extract | 30 grams |
| Cycloheximide | 0.01 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 mL |
Yeast Growth Media
This is a simple non-synthetic yeast growth media shared by Cory Widmayer on Milk The Funk, and is based off of Leite et al. (2013). See Widmayer's post on MTF for directions on using this media (requires aeration; the batch feeding process may not be necessary, see comments by Dr. Bryan Heit). This media and method are claimed to grow Brettanomyces very quickly. Widmayer also provides a synthetic version of the recipe on the MTF post.
| Glucose or another sugar type | 100 grams |
| Yeast Extract | 90 grams |
| Distilled Water | Fill to 1000 mL |
Misc
Storage
- For lactic acid bacteria storage, see Lactobacillus Storage.
- For Brettanomyces storage, see Storing Brettanomyces.
- For S. cerevisiae storage, see Saccharomyces Storage.
Isotonic Sodium Chloride
Isotonic Sodium Chloride is a saline solution which can be used for storage of microbes. Depending on the species, survivability times can range. It should be kept cold just above freezing. Most Saccharomyces strains handle this storage well and can be stored for 2 years like this before needing to re-propagate and re-banking. After one year Lactobacillus and Pediococcus seem to survive moderately well depending on the strain. Brettanomyces however seems to have a harder time maintaining good vitality. Please refer to Justin Amaral's post for information about this.
The solution for Isotonic Sodium Chloride is quite simple. You'll need 9 grams of lab grade Sodium Chloride to 1000 mL of distilled/de-ionized water, autoclaved.
Slants
Creating slants:
Growing cultures from slants:
Growing cultures from slants or plates:
Liquid Slurry
Propagators
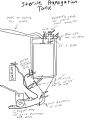
Justin Amaral's 4.5 BBL Propagator
The propagator below has a 14 gallon capacity, allowing a prop up of 4.5 BBL. This same set up can be used on any fermenter but is best used on ones that can hold 5 PSI and up. The seal on the below fermenter can hold around 5 PSI but clamps are also used on the lid to ensure it can hold up to 10 PSI. It essentially acts as a large stir plate while allowing to trickle in either O2, NO2 or CO2 depending on what your propagating. Ideally you'd also use a non magnetic drive pump but its not essential.
Below is the draw up of the build out for this prop up tank. As you can see it uses quick disconnects but you can use tri-clover connects as well. It also uses a diffusion stone that connects into one of the ferrules allowing you to trickle in gases. The picture shows a diffusion stone directly into the fermenter but its since been adapted as shown in the other pictures below to allow the diffusion stone to be behind a valve so its only exposed when needed. It also uses a whirlpool connector that goes into the fermenter creating the whirlpool when the pump is on:
Techniques
(Videos provided by Bryan of Sui Generis blog and Zach Taggart of 42 North Brewing Company.)
Aseptic Technique
Making Agar Plates
- Making WLD plates:
- Making a starter from slants or plates:
Yeast/Bacteria Isolation
- Escarpment Labs agar plating guide.
- Siebel presentation on detecting brewery contamination with agar plating.
- Mark Trent details his method for isolating Brettanomyces from Saccharomyces from dregs or other mixed cultures.
- Ruth Barry details her process for isolating individual strains in a mixed culture with the goal of characterizing each strain for a more controlled mixed fermentation.
- Cory Widmayer's guide to yeast isolation and media growth techniques, with an example of S. pombe and tips for Brettanomyces, Z. bailii, and others.
- See Wild Yeast Isolation.
- See isolating Lactobacillus.
Making Your Own Media
Although media can be bought pre-made, you can also make these media yourself. Media can be either sterilized via an autoclave/pressure cooker or using sterile filtering. Keep in mind it can be difficult however to sterile filter some items such as yeast extract, peptone and brewer's grade DME. Because of this some just autoclave parts of the media while sterile filtering the rest into the sterilized media.
It is crucial you use aseptic technique with your media once it is sterilized to prevent any contamination. If you are storing extra media for later use make sure to remake it every month or 2 if unused.
Yeast Banking
Cell Counting
- Cell counting:
- Cell Density Equation:
- Semi-Automated Cell Counting:
Various other tips:
- Escarpment Labs guide to yeast counting.
- MTF thread with tips on using "cheap Chinese" hemocytometers for cell counting.
- MBAA podcast episode 113 with Bill Maca on how to properly prepare a yeast sample for counting.
Gram Staining
Propagating Yeast
- Propagation techniques:
- "Yeast Propagation at Urban Chestnut" interview with Florian Kuplent of Urban Chestnut on MBAA Podcast episode #239.
- "Small Craft Brewery Yeast Management," by Michael Tonsmeire.
Various other tips:
- When propagating yeast in an Erlenmeyer flask, use a flask that allows for plenty of headspace. This allows for a thinner layer of foam and thus more oxygen diffusion. Set the stir plate or shaker to the highest speed that doesn't produce a lot of foam. A good general rule of thumb is to use a flask that allows for 20-33% starter volume and 66-80% headspace (ideally 20% starter volume and 80% headspace). For example, for a 1L starter, use a 3L, 4L, or 5L flask (ideally 5L). More volume can be used with a Fernbach or a baffled flask, but these require a shaker table. When propagating yeast for use in beer, use wort and a magnesium and zinc nutrient for best yeast propagation practices. Cover the flask with foil (or foam stoppers if fruit flies are a problem) [17].
Semi-Anaerobic Containers for Incubating Plates
Anerobic chambers can be costly. Here is are two DIY methods. The first achieves under 1% O2, and both methods will also prevent mold growth.
- The plating setup: Use 60mm plates; bigger plates might not fit.
- The anaerobic tools: short wide-mouth jam jar, rubber-lined lid.
- After inoculating, loosely tape the lids in place, just so they don't fall off when placing them in the jar.
- Purge the jar with CO2 for 5-10 seconds with lid just ajar.
- Place 1 or 2 Oxy-Sorb 100-Pack Oxygen Absorber, 100cc into the jar.
- Seal the jar with electrical tape.
- Incubate!
Afterwards, seal the package of Oxy-Sorb with a vacuum sealer. Otherwise they will begin to absorb oxygen [18].
An alternative method using a vacuum sealer and a jar lid adapter:
- The plating setup. Use 60mm plates; bigger plates might not fit.
- The anaerobic tools: short wide-mouth jam jar, lid, vacuum sealer, ball jar attachment.
- After inoculating, loosely tape the lids in place, just so they don't fall off when placing them in the jar.
- Purge the container with CO2 for 5-10 seconds with lid just ajar.
- Place ball jar adapter on jar and vacuum seal container.
- Incubate!
These methods were shared by Bryan of Sui Generis Blog and Zach Taggart of 42 North Brewing Co. See this MTF thread for details.
Identification
- "Basic Yeast Morphology" by Dr. Bryan Heit of Sui Generis Blog.
- Richard Preiss explains the differences between ITS sequencing, PCR fingerprinting, Low coverage whole genome short read sequencing, Higher coverage whole genome short read sequencing, and Long read sequencing (PacBio), as well as why some methods have a high cost.
- MTF thread on methods for identifying strain level differences.
- Identifying Wild Yeast.
Yeast Rinsing/Washing
Rising:
Acid washing:
Hybridization and Modification
Shipping Cultures
DeWayne Schaaf recommends the following procedures for shipping cultures with the USA states and territories [19]:
- Use 15ml centrifuge tubes from Cynmar LLC - Wine & Brew. They are great quality and have a very low rate of leakage, especially when combined with electrical tape.
- Place each liquid filled tube into its own snack sized baggie to minimize cross-contamination if they do happen to leak.
- Each of these smaller bags will be placed in a quart Ziplock.
- My main shipping container is Uline Poly bubble mailers. I don't tend to use ice packs as I've found them unnecessary when shipping in favorable temps (use ice packs during the summer).
- USPS is my preferred shipping method. Using first class shipping, my packages typically take no more than 3 days to reach somewhere in the USA and cost right around $3, and that includes a tracking number. Shipping rates are roughly the same for any USA state or territory. The 48 continental states, Alaska, Hawaii, Puerto Rico, and Guam all cost roughly the same.
For 5 gallon pitches or cultures that may still be a bit active creating cO2 Soda Preform tubes work very well. These are the same type of tubes White Labs uses for their homebrew pitches. https://www.amazon.com/Soda-Bottle-Preforms-Caps-30/dp/B008MB1QNY/ref=sr_1_1?ie=UTF8&qid=1504524577&sr=8-1&keywords=soda+test+tubes
See also:
- Bryan of Sui Generis Blog guide to shipping yeast (recommended for larger quantities).
- Labeling recommendations for intercontinental shipping.
Laboratory Information Management Systems
- Brewery Pi (open source, but still in development; sponsored by Deschutes).
Chemistry
Historical
- "Pasteur and the beer of national revenge", by Lars Garshol (how Louis Pasteur proved that microbes were alive in 1857, and other discoveries).
- "Emil Chr. Hansen and the yeast revolution", by Lars Garshol (how yeast was first isolated from other strains in 1883).
- Characterization of Old Wine Yeasts Kept for Decades under a Zero-Emission Maintenance Regime.
See Also
Additional Articles on MTF Wiki
- Quality Assurance
- Microscope
- PH Meter
- Wild Yeast Isolation
- Titratable Acidity
- Saccharomyces
- Brettanomyces
- Pediococcus
- Lactobacillus
External Resources
- ASBC Webinar: Green Chemistry & Beer Science by Dana Garves of Oregon BrewLab.
- Bootleg Biology: a Semester-Long CURE Using Wild Yeast to Brew Beer.
- "Yeast: The Practical Guide to Beer Fermentation," by: Chris White and Jamil Zainasheff.
- BasicBrewing interview with Zack Taggart, lab manager at 42 North Brewing, on how to set up our own homebrew lab to analyze yeast health and count cell population (part 1) and isolating, propagating, and storing yeast and bacteria (part 2).
- "Brewers' Laboratory Handbook: Brewing Without the Blindfold™", by Brewing Science Institute.
- "Selective Media Part 1" by BKYeast blog.
- "Pouring Plates and Making Slants" by BKYeast blog.
- American Society of Brewing Chemists.
- Eureka Brewing Blog on lab techniques, yeast handling, etc.
References
- ↑ Yakobson, Chad. The Brettanomyces Project. Propagation and Batch Culture Methods. Retrieved 2/18/2015.
- ↑ Nick Impellitteri. Private correspondence with Dan Pixley. 03/23/2020.
- ↑ Richard Preiss. Milk The Funk Facebook group thread about making agar. 08/27/2019.
- ↑ Christophe Pichon. Milk The Funk Facebook post regarding acetic acid bacteria isolation. 06/30/2021.
- ↑ Richard Preiss, Justin Amaral, and Lance Shaner. Milk The Funk Facebook thread on detecting Brettanomyces in a Saccharomyces culture. 01/18/2018.
- ↑ Development of detection medium for hard-to-culture beer-spoilage lactic acid bacteria. Suzuki K, Asano S, Iijima K, Kuriyama H, Kitagawa Y. 2008.
- ↑ 125th Anniversary Review: Microbiological Instability of Beer Caused by Spoilage Bacteria. Koji Suzuki. 2011.
- ↑ Nick Impellitteri. Milk The Funk Facebook group post on his Lactobacillus growth media formulation. 03/20/2020.
- ↑ Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. F.A. Cubillos, B. Gibson, N. Grijalva‐Vallejos, K. Krogerus, J. Nikulin. 2019. DOI: https://doi.org/10.1002/yea.3380.
- ↑ Himedia LCSM technical Data Sheet. Retrieved 01/04/2019.
- ↑ Shawn Savuto. Milk The Funk Facebook group post on Escarpment Labs CSSM. 05/05/2022.
- ↑ Effects of Beer Adaptation on Culturability of Beer-Spoilage Dekkera/Brettanomyces Yeasts. Koji Suzuki, Shizuka Asano, Kazumaru Iijima, Tomoo Ogata, Yasushi Kitagawa & Tsunehiro Ikeda. 2018.
- ↑ Richard Preiss. Milk The Funk Facebook group thread on DBDM medium. 03/01/2019.
- ↑ Development of an enrichment medium to detect Dekkera/Brettanomyces bruxellensis, a spoilage wine yeast, on the surface of grape berries. Vincent Renouf, Aline Lonvaud-Funel. 2007. DOI: https://doi.org/10.1016/j.micres.2006.02.006.
- ↑ Occurrence of Brettanomyces bruxellensis on grape berries and in related winemaking cellar. Francesca Comitini1, Lucia Oro, Laura Canonico, Valentina Marinelli, Maurizio Ciani. 2019. DOI: 10.3389/fmicb.2019.00415.
- ↑ van Wyk, S.; Silva, F.V.M. Enumeration of Brettanomyces in Wine Using Impedance. Appl. Microbiol. 2021, 1, 352-360. https://doi.org/10.3390/applmicrobiol1020024.
- ↑ Various MTF members. Milk The Funk Facebook thread started by Jon Stanley on Erlenmeyer flask usage for yeast starters. 02/12/2018.
- ↑ Bryan of Sui Generis Blog and Caroline (Zach) Taggart on DIY semi-aerobic environment for plating. Milk The Funk Facebook group. 10/05/2017.
- ↑ DeWayne Schaaf. Kveik World Order Facebook page. 04/21/2017. Retrieved 04/24/2017.