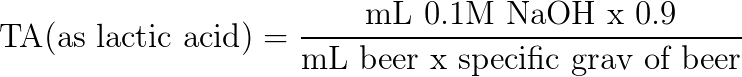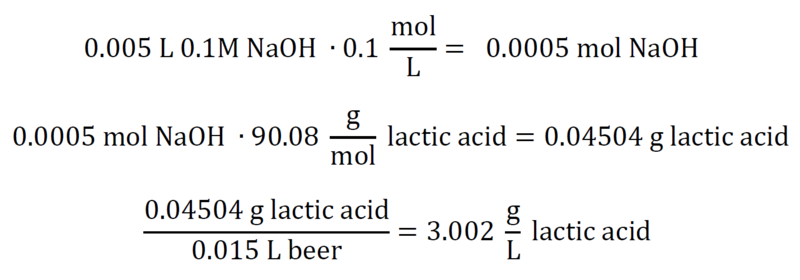Difference between revisions of "Titratable Acidity"
m |
(→External Resources) |
||
| (69 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Titratable Acidity''' (abbreviated as '''TA''') is an approximation of the ''Total Acidity'' of a solution, and has long been used in the production of wine. It is usually expressed in units of grams per liter (g/L), although other formats are also used <ref>[http://www.accuvin.com/wp-content/uploads/2015/04/Monitoring-Acids-and-pH-in-Winemaking.pdf Wine From the Outside - Easy Wine Chemistry For the Casual Chemist. Monitoring Acids and pH in Winemaking. Mike Miller.]</ref>. Titratable Acidity is often mistakenly confused with Total Acidity, but they are not the same thing | + | '''Titratable Acidity''' (abbreviated as '''TA''') is an approximation of the ''Total Acidity'' of a solution, and has long been used in the production of wine. It is usually expressed in units of grams per liter (g/L), although other formats are also used <ref>[http://www.accuvin.com/wp-content/uploads/2015/04/Monitoring-Acids-and-pH-in-Winemaking.pdf Wine From the Outside - Easy Wine Chemistry For the Casual Chemist. Monitoring Acids and pH in Winemaking. Mike Miller.]</ref>. Titratable Acidity is often mistakenly confused with Total Acidity, but they are not the same thing <ref>[http://wineserver.ucdavis.edu/pdf/attachment/220%20relationship%20between%20total%20acidity,%20TA,%20and%20pH%20.pdf The relationship between total acidity, titratable acidity and pH in wine. Roger Boulton. American Journal of Enology and Viticulture. 31(1): 76-80. 1980.]</ref>. While Total Acidity is a more accurate measurement of the total acid content of a solution, Titratable Acidity is used because it is easier to measure. Although titratable acidity does not measure all acids, TA is generally considered a better way to measure perceivable acidity in sour beer and cider than pH <ref>[http://blog.ocbeerblog.com/2015/04/13/how-sour-is-your-sour-beer/ How Sour is Your Sour Beer?. OCBeerBlog on Firestone Walker's demonstration of the uses of TA measurements. April 13, 2015.]</ref><ref>[http://www.cider.org.uk/phandacid.htm "Relationship between pH and Titratable acid in Cider Apple Juices." The Wittenham Hill Cider Portal (blog). 2011. Retrieved 11/24/2017.]</ref>. |
==TA versus pH== | ==TA versus pH== | ||
| Line 5: | Line 5: | ||
===pH=== | ===pH=== | ||
| − | In chemistry, pH is the negative log of the activity of the hydrogen ion (H+) in an aqueous solution. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline. Pure water has a pH of 7. | + | In chemistry, pH is the negative log of the activity of the free (dissociated) hydrogen ion (H+) in an aqueous solution. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline. Pure water has a pH of 7. |
The pH scale is traceable to a set of standard solutions whose pH is established by international agreement <ref name="bates">Bates, Roger G. Determination of pH: theory and practice. Wiley, 1973.</ref>. Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode. Measurement of pH for aqueous solutions can be done with a glass electrode and a pH meter, or using indicators. | The pH scale is traceable to a set of standard solutions whose pH is established by international agreement <ref name="bates">Bates, Roger G. Determination of pH: theory and practice. Wiley, 1973.</ref>. Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode. Measurement of pH for aqueous solutions can be done with a glass electrode and a pH meter, or using indicators. | ||
| Line 11: | Line 11: | ||
pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications <ref name="bates"></ref>. | pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications <ref name="bates"></ref>. | ||
| − | pH is best tested in sour beers using a [[ | + | pH is best tested in sour beers using a [[pH Meter]] and is most useful for biological parameters. Microbial growth, vitality, and death are evaluated based on pH rather than TA. This means pH should be used when testing sanitizer, [[Wort Souring]], starter cultures, etc. |
| − | === | + | ===TA=== |
| − | + | Titration is an attempt to quantify an unknown substance with a known one. Titratable acidity asks how much of a given base (in our case sodium hydroxide, NaOH) neutralizes the acid(s) (lactic, phosphoric, etc.) in a volume of liquid, thus estimating both free hydrogen ions and hydrogen ions that are bound to weak acids that can react with the strong base and be neutralized <ref>[https://www.umpqua.edu/images/areas-of-study/career-technical/viticulture-enology/downloads/conferences/technical-symposia/2014-dec-wine-chemistry/2014-ts-3-ph-ta-n2.pdf Gump, Barry H. "pH and Titratable Acidity" Presentation. 2014. Retrieved 11/14/2017.]</ref>. The units of TA can be quoted in g/L, or in other words, so many grams (of a specific acid) in so much substrate (beer) brings the pH of that substrate to a predetermined pH (for instance, a pH of 7 or 8.2). | |
| − | + | Titratable acidity does not target a specific acid in the liquid you are measuring. Beer is composed of lactic acid, but also phosphoric acid, acetic acid, etc. While the latter are in minute quantities, they still affect the end result. For our purposes (and convention), we assume 100% lactic acid in the sample for our titration. | |
| − | + | Why care about titratable acidity? pH quantifies the number of free hydrogen ions (or hydrogen ion equivalents) in liquid. Your palate does not measure pH directly. Your palate interprets a multi-variable substrate called beer, which also contains "weak acids" such as lactic acid that contain bonded hydrogen ions that are not measured by pH. Titratable acidity attempts to put another quantifiable handle on your beer akin to pH; the measurement better captures how “acidic” the beer may taste to you. Again, there are other acids than lactic in the beer, leading to differences in flavor between beers of the same TA. As a general rule of thumb, the lower the pH, the higher the TA, however, TA and pH are not directly correlated. TA has been proposed as a more accurate representation of perceived acidity because we taste both free hydrogen ions and those that are bound to organic acids <ref>[http://www.craftbrewersconference.com/wp-content/uploads/2015_presentations/W1320_Kara_Taylor.pdf Kara Taylor. CBC 2015 Presentation. 2015. Retrieved 11/14/2017.]</ref><ref>[http://www.cider.org.uk/phandacid.htm The Wittenham Hill Cider Portal. "Relationship between pH and Titratable acid in Cider Apple Juices". Retrieved 11/14/2017.]</ref>. There is some disagreement on whether TA is a more accurate measurement of perceived acidity than pH; see [[Titratable_Acidity#Limitations_of_TA|Limitations of TA]] for details. | |
| − | + | Titratable acidity can be expressed in terms of different acids. In wine, TA is generally expressed in terms of tartaric acid (molecular weight of 150.09). In sour beer, TA is expressed in terms of lactic acid (molecular weight 90.08, which is where the "0.9" number comes from in the equation below). To express TA in terms of a specific acid, the molecular weight of the specified acid is used in the TA calculation. In the [[Titratable_Acidity#Example|example below]], we express the TA value in terms of lactic acid. See [http://www.awri.com.au/wp-content/uploads//TN14.pdf appendix 1 in this paper] on how to convert the titratable acidity value for different acids. Note that this is NOT a measurement of how much lactic acid or tartaric acid there is, it is an expression of measurement like how feet and meters are two different expressions of measurement for the same thing (distance). For example, a TA of 5.0 measured in units of tartaric acid is equal to a TA 6.0015 measured in units of lactic acid. Therefore, an argument can be made that TA measurements should always be specified as to which acid was used in the calculation. | |
| − | |||
| − | = | + | The ASBC<ref name="ASBC" /> equation for TA in units of lactic acid (the 0.9 constant represents TA expressed in units of lactic acid, also called the ''correction factor'') is as follows: |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | [[File:TA Formula.gif|center|Titratable Acidity Example]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
===Example=== | ===Example=== | ||
''"I agree that maths are hard."'' - Lance Shaner. | ''"I agree that maths are hard."'' - Lance Shaner. | ||
| − | + | What you will need: | |
| − | |||
| − | |||
| − | : | ||
| − | |||
| − | |||
| − | |||
| − | + | * A reliable and calibrated [[pH Meter]]. | |
| − | |||
| − | * [ | ||
| − | + | * Sample of beer to be measured. Must be fully degassed if it has any carbonation (pour through a coffee filter, or shake and ventilate to decarbonate). | |
| − | * | + | |
| − | + | * Sodium Hydroxide, NaOH. Available in liquid or powder form. Be sure to note its molarity (M), units of mol/L. For more info on mol, see [https://en.wikipedia.org/wiki/Mole_(unit) here]. | |
| + | |||
| + | :*<code>'''Safety caution''': always wear safety glasses and gloves when handling NaOH in any concentration. NaOH can cause severe burns. In concentrations higher than 0.1, NaOH can corrode through clothing. See [https://www.ccohs.ca/oshanswers/chemicals/chem_profiles/sodium_hydroxide.html Canadian Centre for Occupational Health and Safety on Sodium Hydroxide].</code> | ||
| + | |||
| + | :*Note that bottles of NaOH should be capped so as to avoid as much exposure to the air as possible. This is because NaOH reacts with CO<sub>2</sub> in the air, causing the NaOH to become inaccurate over time as a base titration <ref>[https://www.sciencedirect.com/science/article/pii/S2405656116300578 A novel rate of the reaction between NaOH with CO<sub>2</sub> at low temperature in spray dryer. Yadollah Tavan, Seyyed Hossein Hosseini. 2017. DOI: https://doi.org/10.1016/j.petlm.2016.11.006.]</ref><ref>[https://www.chem.indiana.edu/wp-content/uploads/2017/11/17-3-Sodium-Hydroxide-and-Carbon-Dioxide.doc "Sodium Hydroxide and Carbon Dioxide: Why it is Important to Keep Your Standard NaOH Solutions Capped". Indiana University course material. Retrieved 10/18/2018.]</ref><ref>[https://www.homebrewersassociation.org/how-to-brew/resources/conference-seminars Robert Hall. The "Sour" in Sour Beers: Microbiology Sensory Perception and Styles. HomebrewCon 2018 Seminar. 2018.]</ref> (~42:00 mins in). | ||
| + | |||
| + | * Nitrile or latex gloves. NaOH is a strong base, it will hurt you if you get any on your skin. | ||
| + | |||
| + | * Pipettes and glassware, with precision down to 0.1 mL (25 or 50mL buret <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1903175736377298/ Various MTF members. Milk The Funk thread on what size buret to use for titrations. 12/01/2017.]</ref>). Alternatively, you can use a precision scale to dose the base into the beer, if you know the density of both liquids (preferred method). | ||
| + | |||
| + | We need a precise volume of the beer and the specific gravity of the beer. If the specific gravity cannot be measured easily for some reason, then estimating it will be fine for this equation since small differences in specific gravity don't greatly impact the results of the equation. In this example, we have 15 mL with a specific gravity of 1.015. We also need NaOH in liquid form. Typically, it is sold in 0.1M form. Now, the trickiest part of this is adding precise amounts of NaOH (say, 0.1-0.5 mL at time), to your 15 mL of beer. Every time you add NaOH, you must vigorously stir the sample so it is well-mixed. Then you can measure its pH. You continue this until you reach the desired pH baseline of 8.2. | ||
| + | |||
| + | :''Note: The baseline value of 8.2 pH is somewhat arbitrary, but it is the US (ASBC<ref name="ASBC">[http://methods.asbcnet.org/summaries/beer-8.aspx ASBC Methods of Analysis. Total Acidity. Retrieved 11/8/2017.]</ref>) and Australian industry standard. A pH of 7 is a neutral pH and the pH of water, whereas ~8.2 is near the equivalence point for a lactic acid/sodium hydroxide reaction. A pH of 8.2 is also where a titration dye, phenolphthalein, changes color. A well-calibrated pH meter is easier to use than dye, not to mention its superior accuracy and precision, if used correctly (well-calibrated, probe well-maintained, etc.). A pH of 7 is the European industry standard for measuring TA in wine <ref>[http://www.awri.com.au/wp-content/uploads//TN14.pdf "TN14 - Interconversion of acidity units" Industry Development and Support. Australian Wine Research Institute. Retrieved 09/15/2016.]</ref>. See also [https://www.facebook.com/groups/MilkTheFunk/permalink/1877187898976082/ this MTF thread].'' | ||
| + | |||
| + | At or around a pH of 8.2, we have reached our equivalence point for a titration of pure NaOH and pure lactic acid. We need to convert the moles of NaOH we added into moles of lactic acid, and then divide the equivalent grams of lactic acid by the original volume of beer. That gets us g/L, and our titratable acidity. For a numerical example, assume '''15mL''' beer with a gravity of '''1.015''', '''5mL''' 0.1M NaOH: | ||
| + | |||
| + | |||
| + | [[File:TA example.gif|center|Titratable Acidity Example]] | ||
| + | |||
| + | |||
| + | In summary, the measurement of titratable acidity is a technique to quantify the total acid level of a beer. A major assumption was made: all the acid in the liquid was lactic acid. Two beers could have the same TA measurement, but have differing levels of palatable acidity, due to the acid makeup of the beer. | ||
| + | |||
| + | ===Eccentric Beekeeper TA Calculator=== | ||
| + | The [https://docs.google.com/spreadsheets/d/1DR48ivi9xSoKOl3TmsaqhWg8yLlcP6BgO5eKZ-suITo/edit?usp=sharing Eccentric Beekeeper TA Spreadsheet] (save a copy to your local computer to use it) calculates TA as well as blends of beers with different TA values. It also includes a correction for beer final gravity. The idea is that the more residual sugar there is the less effect the acid will have on your perception. This is likely not that straightforward since you can have varying levels of sweetness at the same given FG <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1097532690274944/?comment_id=1097668506928029&offset=0&total_comments=19&comment_tracking=%7B%22tn%22%3A%22R5%22%7D Conversation with Dave Janssen on MTF. 6/23/2015.]</ref>. | ||
| + | |||
| + | ===ASBC Modification Using Mass=== | ||
| + | Here, MTF member Andy Carter presents an alternative calculation for measuring titratable acidity that allows for easier accuracy given lower than lab quality instrumentation. The ASBC method requires an accurate measurement of both beer volume and density. Depending on the quality of lab equipment available, these measurements can have variable error bars. In general, more accurate mass scales are more affordable than similarly accurate volume or density instruments, so only using the beer and acid mass will lead to a more consistent measurement. Additionally, small gravity differences don't have a large impact when using the ASBC calculation. Therefore, the following calculation for TA does not use volume or specific gravity. | ||
| + | |||
| + | For a numerical example of this alternative calculation for TA, assume 15mL beer, 5mL 0.1M NaOH: | ||
| + | |||
| + | [[File:TA_calc.PNG|center|800px|Titratable Acidity Example]] | ||
| + | |||
| + | ==Videos== | ||
| + | [https://www.khanacademy.org/science/chemistry/acid-base-equilibrium/titrations/v/titration-of-a-weak-acid-with-a-strong-base Titration of a weak acid with a strong base by Kahn Acadmey part 1:] | ||
| + | |||
| + | <youtube height="200" width="300">x3CbfUr449Y</youtube> | ||
| + | |||
| + | [https://www.khanacademy.org/science/chemistry/acid-base-equilibrium/titrations/v/titration-of-a-weak-acid-with-a-strong-base-continued Part 2:] | ||
| + | |||
| + | <youtube height="200" width="300">WbDL7xN-Pn0</youtube> | ||
| + | |||
| + | Dana Garves from Oregon BrewLab presentation on pH vs TA: | ||
| + | |||
| + | <youtube height="200" width="300">_iQFRRTLBPc</youtube> | ||
| + | |||
| + | ==Limitations of TA== | ||
| + | Although a better representation of perceived acidity than pH, titratable acidity has some limitations on accurately measuring perceived acidity in beer. [[Omega_Yeast_Labs|Omega Yeast]] performed [https://topcrop.co/dialing-in-sourness-ph-vs-ta an experiment] to compare pH and TA. While TA represented sensory test results of which samples were more sour than others in many cases, there were some exceptions. For example, beers dosed with the same amount of acetic acid versus lactic acid measured the same TA, but the acetic acid sample measured higher in sour flavor in the sensory test. Different types of beers dosed with the same amount of acid measured the same TA, but they had different sourness levels in the sensory test. Also, beers dosed with double the amount of acid did not necessarily taste twice as sour, meaning that the taste of sourness is not linear with higher TA levels. Omega Yeast concluded that the types of acids can greatly impact the sensory experience of sourness, as well as the base beer. While TA can be a good indicator of sourness, it still cannot replace the human organoleptic system. | ||
| + | |||
| + | See also: | ||
| + | * [https://topcrop.co/dialing-in-sourness-ph-vs-ta "Dialing in Sourness: pH vs TA" by Laura Burns and Shana Solarte.] | ||
| + | * [https://www.facebook.com/groups/MilkTheFunk/permalink/1481798761848333/ MTF discussion on TA]. | ||
| + | |||
| + | ==Lactic Acid Test== | ||
| + | Tim Lozen from Bells Brewing Co reported in a presentation for the Master Brewers Conference that using a [https://www.sigmaaldrich.com/catalog/product/mm/116127?lang=en®ion=US Lactic Acid Test] using reflectometric with test strips (RQ) to test the amount of lactic acid was cheaper than other laboratory methods such as chromatography. They reported that the RQ measurement was more relevant to perceived acidity, and proposed that a ratio of RQ to TA resulted in the consistent production of sour beer (presumably kettle sours) <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/2074383085923228/ Dan Pixley. Milk The Funk Facebook group post that summarizes the MBAA Podcast episode 85. 04/29/2018.]</ref>. See [http://masterbrewerspodcast.com/085-lactic-acid-bacteria-case-study this MBAA podcast episode]. | ||
==See Also== | ==See Also== | ||
| Line 71: | Line 95: | ||
===External Resources=== | ===External Resources=== | ||
| − | * More information on this procedure is available from the [http:// | + | * [http://tinyurl.com/1iv70kjf ph Meter and TA kit equipment list with prices and links by Oregon BrewLab.] |
| + | * [http://braukaiser.com/blog/blog/2010/11/25/wort-and-beer-titration/ Wort and Beer Titration by Kai Troester.] | ||
| + | * More information on this procedure is available from the [http://methods.asbcnet.org/summaries/beer-8.aspx American Society of Brewing Chemists], who publish a similar set of procedures under the name "Total Acidity with Potentiometer". | ||
* [http://www.mbaa.com/districts/Northwest/mash/Documents/Acidity%20and%20Blending.pdf Jim Crooks of Firestone Walker presentation about blending sour beers using TA]. | * [http://www.mbaa.com/districts/Northwest/mash/Documents/Acidity%20and%20Blending.pdf Jim Crooks of Firestone Walker presentation about blending sour beers using TA]. | ||
* [http://horscategoriebrewing.blogspot.com/2015/05/cbc-take-homes.html Dave Janssen's discussion of Kara Taylor's CBC talk]. | * [http://horscategoriebrewing.blogspot.com/2015/05/cbc-take-homes.html Dave Janssen's discussion of Kara Taylor's CBC talk]. | ||
* [http://embracethefunk.com/ph-readings-of-commercial-beers/ pH Readings of Commercial Beers, Embrace the Funk Blog, Brandon Jones.] | * [http://embracethefunk.com/ph-readings-of-commercial-beers/ pH Readings of Commercial Beers, Embrace the Funk Blog, Brandon Jones.] | ||
| + | * [https://beerandbrewing.com/understanding-ph-and-titratable-acidity-in-sour-beer-tools-for-brewers-and/ "Understanding pH and Titratable Acidity in Sour Beer: Tools for Brewers and Enthusiasts Alike" by Stan Hieronymus on the Craft Beer & Brewing Magazine website.] | ||
* [http://www.amazon.com/gp/product/B016AY7T76?colid=2U7JPR40PC5G2&coliid=I30J7IAENBH9DZ Amazon source for NaOH.] | * [http://www.amazon.com/gp/product/B016AY7T76?colid=2U7JPR40PC5G2&coliid=I30J7IAENBH9DZ Amazon source for NaOH.] | ||
| − | * [https://www.facebook.com/groups/MilkTheFunk/permalink/ | + | * [https://www.facebook.com/groups/MilkTheFunk/permalink/1211858545509024/ MTF discussion regarding Kara Taylor's BA presentation that shows TA for multiple beers, and suggestion for using "Sour Units" as a measurement for beer.] |
* [https://www.facebook.com/groups/MilkTheFunk/permalink/1228704607157751/?comment_id=1228977910463754&reply_comment_id=1228997810461764&comment_tracking=%7B%22tn%22%3A%22R7%22%7D MTF tips on safety and methods of measuring TA from a research technician.] | * [https://www.facebook.com/groups/MilkTheFunk/permalink/1228704607157751/?comment_id=1228977910463754&reply_comment_id=1228997810461764&comment_tracking=%7B%22tn%22%3A%22R7%22%7D MTF tips on safety and methods of measuring TA from a research technician.] | ||
| + | * [https://www.beeradvocate.com/mag/14649/savoring-acidity-the-quest-to-explain-sourness-in-beer/?mc_cid=16534dd7fd&mc_eid=243e286e71 "Savoring Acidity: The Quest to Explain Sourness in Beer", by Brian Yaeger in BeerAdvocate magazine.] | ||
| + | * [http://sourbeerblog.com/a-guide-to-blending-sour-beer-with-fruit/ "A Guide to Blending Sour Beer With Fruit" by Matt Miller; includes ABV and TA calculators for fruit additions.] | ||
| + | * [https://www.beyersanalytical.com/redcheck RedCheck Titratable Acidity kit.] <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1934289469932591/?comment_id=1944943422200529&reply_comment_id=1945120492182822&comment_tracking=%7B%22tn%22%3A%22R1%22%7D Kelley Freeman. Milk The Funk Facebook group thread on TA kits. 01/06/2018.]</ref> | ||
| + | * [https://www.lallemandbrewing.com/en/canada/brewers-corner/brewing-tools/measuring-titratable-acidity Lallemand Brewing online TA tool and instructions using ASBC methods.] | ||
| + | * [https://blueowlbrewing.com/2017/06/20/what-is-a-sour-unit Blue Owl Brewing uses an altered TA number called "Sour Units" to communicate to customers how acidity their beers are.] | ||
| + | |||
| + | ==Authorship== | ||
| + | |||
| + | Originally written by James Howat with major updates by Andy Carter, and with input from Dan Pixley and organic chemist Mike Castagno. | ||
==References== | ==References== | ||
Latest revision as of 20:24, 13 June 2024
Titratable Acidity (abbreviated as TA) is an approximation of the Total Acidity of a solution, and has long been used in the production of wine. It is usually expressed in units of grams per liter (g/L), although other formats are also used [1]. Titratable Acidity is often mistakenly confused with Total Acidity, but they are not the same thing [2]. While Total Acidity is a more accurate measurement of the total acid content of a solution, Titratable Acidity is used because it is easier to measure. Although titratable acidity does not measure all acids, TA is generally considered a better way to measure perceivable acidity in sour beer and cider than pH [3][4].
Contents
TA versus pH
Many sour beer producers use pH to help determine how "sour" their beer is in relation to a set goal, previous batches, or commercial examples. However, often times TA is a more accurate measurement of how acidic a beer will be perceived on the palate.
pH
In chemistry, pH is the negative log of the activity of the free (dissociated) hydrogen ion (H+) in an aqueous solution. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline. Pure water has a pH of 7.
The pH scale is traceable to a set of standard solutions whose pH is established by international agreement [5]. Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode. Measurement of pH for aqueous solutions can be done with a glass electrode and a pH meter, or using indicators.
pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications [5].
pH is best tested in sour beers using a pH Meter and is most useful for biological parameters. Microbial growth, vitality, and death are evaluated based on pH rather than TA. This means pH should be used when testing sanitizer, Wort Souring, starter cultures, etc.
TA
Titration is an attempt to quantify an unknown substance with a known one. Titratable acidity asks how much of a given base (in our case sodium hydroxide, NaOH) neutralizes the acid(s) (lactic, phosphoric, etc.) in a volume of liquid, thus estimating both free hydrogen ions and hydrogen ions that are bound to weak acids that can react with the strong base and be neutralized [6]. The units of TA can be quoted in g/L, or in other words, so many grams (of a specific acid) in so much substrate (beer) brings the pH of that substrate to a predetermined pH (for instance, a pH of 7 or 8.2).
Titratable acidity does not target a specific acid in the liquid you are measuring. Beer is composed of lactic acid, but also phosphoric acid, acetic acid, etc. While the latter are in minute quantities, they still affect the end result. For our purposes (and convention), we assume 100% lactic acid in the sample for our titration.
Why care about titratable acidity? pH quantifies the number of free hydrogen ions (or hydrogen ion equivalents) in liquid. Your palate does not measure pH directly. Your palate interprets a multi-variable substrate called beer, which also contains "weak acids" such as lactic acid that contain bonded hydrogen ions that are not measured by pH. Titratable acidity attempts to put another quantifiable handle on your beer akin to pH; the measurement better captures how “acidic” the beer may taste to you. Again, there are other acids than lactic in the beer, leading to differences in flavor between beers of the same TA. As a general rule of thumb, the lower the pH, the higher the TA, however, TA and pH are not directly correlated. TA has been proposed as a more accurate representation of perceived acidity because we taste both free hydrogen ions and those that are bound to organic acids [7][8]. There is some disagreement on whether TA is a more accurate measurement of perceived acidity than pH; see Limitations of TA for details.
Titratable acidity can be expressed in terms of different acids. In wine, TA is generally expressed in terms of tartaric acid (molecular weight of 150.09). In sour beer, TA is expressed in terms of lactic acid (molecular weight 90.08, which is where the "0.9" number comes from in the equation below). To express TA in terms of a specific acid, the molecular weight of the specified acid is used in the TA calculation. In the example below, we express the TA value in terms of lactic acid. See appendix 1 in this paper on how to convert the titratable acidity value for different acids. Note that this is NOT a measurement of how much lactic acid or tartaric acid there is, it is an expression of measurement like how feet and meters are two different expressions of measurement for the same thing (distance). For example, a TA of 5.0 measured in units of tartaric acid is equal to a TA 6.0015 measured in units of lactic acid. Therefore, an argument can be made that TA measurements should always be specified as to which acid was used in the calculation.
The ASBC[9] equation for TA in units of lactic acid (the 0.9 constant represents TA expressed in units of lactic acid, also called the correction factor) is as follows:
Example
"I agree that maths are hard." - Lance Shaner.
What you will need:
- A reliable and calibrated pH Meter.
- Sample of beer to be measured. Must be fully degassed if it has any carbonation (pour through a coffee filter, or shake and ventilate to decarbonate).
- Sodium Hydroxide, NaOH. Available in liquid or powder form. Be sure to note its molarity (M), units of mol/L. For more info on mol, see here.
Safety caution: always wear safety glasses and gloves when handling NaOH in any concentration. NaOH can cause severe burns. In concentrations higher than 0.1, NaOH can corrode through clothing. See Canadian Centre for Occupational Health and Safety on Sodium Hydroxide.
- Nitrile or latex gloves. NaOH is a strong base, it will hurt you if you get any on your skin.
- Pipettes and glassware, with precision down to 0.1 mL (25 or 50mL buret [13]). Alternatively, you can use a precision scale to dose the base into the beer, if you know the density of both liquids (preferred method).
We need a precise volume of the beer and the specific gravity of the beer. If the specific gravity cannot be measured easily for some reason, then estimating it will be fine for this equation since small differences in specific gravity don't greatly impact the results of the equation. In this example, we have 15 mL with a specific gravity of 1.015. We also need NaOH in liquid form. Typically, it is sold in 0.1M form. Now, the trickiest part of this is adding precise amounts of NaOH (say, 0.1-0.5 mL at time), to your 15 mL of beer. Every time you add NaOH, you must vigorously stir the sample so it is well-mixed. Then you can measure its pH. You continue this until you reach the desired pH baseline of 8.2.
- Note: The baseline value of 8.2 pH is somewhat arbitrary, but it is the US (ASBC[9]) and Australian industry standard. A pH of 7 is a neutral pH and the pH of water, whereas ~8.2 is near the equivalence point for a lactic acid/sodium hydroxide reaction. A pH of 8.2 is also where a titration dye, phenolphthalein, changes color. A well-calibrated pH meter is easier to use than dye, not to mention its superior accuracy and precision, if used correctly (well-calibrated, probe well-maintained, etc.). A pH of 7 is the European industry standard for measuring TA in wine [14]. See also this MTF thread.
At or around a pH of 8.2, we have reached our equivalence point for a titration of pure NaOH and pure lactic acid. We need to convert the moles of NaOH we added into moles of lactic acid, and then divide the equivalent grams of lactic acid by the original volume of beer. That gets us g/L, and our titratable acidity. For a numerical example, assume 15mL beer with a gravity of 1.015, 5mL 0.1M NaOH:
In summary, the measurement of titratable acidity is a technique to quantify the total acid level of a beer. A major assumption was made: all the acid in the liquid was lactic acid. Two beers could have the same TA measurement, but have differing levels of palatable acidity, due to the acid makeup of the beer.
Eccentric Beekeeper TA Calculator
The Eccentric Beekeeper TA Spreadsheet (save a copy to your local computer to use it) calculates TA as well as blends of beers with different TA values. It also includes a correction for beer final gravity. The idea is that the more residual sugar there is the less effect the acid will have on your perception. This is likely not that straightforward since you can have varying levels of sweetness at the same given FG [15].
ASBC Modification Using Mass
Here, MTF member Andy Carter presents an alternative calculation for measuring titratable acidity that allows for easier accuracy given lower than lab quality instrumentation. The ASBC method requires an accurate measurement of both beer volume and density. Depending on the quality of lab equipment available, these measurements can have variable error bars. In general, more accurate mass scales are more affordable than similarly accurate volume or density instruments, so only using the beer and acid mass will lead to a more consistent measurement. Additionally, small gravity differences don't have a large impact when using the ASBC calculation. Therefore, the following calculation for TA does not use volume or specific gravity.
For a numerical example of this alternative calculation for TA, assume 15mL beer, 5mL 0.1M NaOH:
Videos
Titration of a weak acid with a strong base by Kahn Acadmey part 1:
Dana Garves from Oregon BrewLab presentation on pH vs TA:
Limitations of TA
Although a better representation of perceived acidity than pH, titratable acidity has some limitations on accurately measuring perceived acidity in beer. Omega Yeast performed an experiment to compare pH and TA. While TA represented sensory test results of which samples were more sour than others in many cases, there were some exceptions. For example, beers dosed with the same amount of acetic acid versus lactic acid measured the same TA, but the acetic acid sample measured higher in sour flavor in the sensory test. Different types of beers dosed with the same amount of acid measured the same TA, but they had different sourness levels in the sensory test. Also, beers dosed with double the amount of acid did not necessarily taste twice as sour, meaning that the taste of sourness is not linear with higher TA levels. Omega Yeast concluded that the types of acids can greatly impact the sensory experience of sourness, as well as the base beer. While TA can be a good indicator of sourness, it still cannot replace the human organoleptic system.
See also:
Lactic Acid Test
Tim Lozen from Bells Brewing Co reported in a presentation for the Master Brewers Conference that using a Lactic Acid Test using reflectometric with test strips (RQ) to test the amount of lactic acid was cheaper than other laboratory methods such as chromatography. They reported that the RQ measurement was more relevant to perceived acidity, and proposed that a ratio of RQ to TA resulted in the consistent production of sour beer (presumably kettle sours) [16]. See this MBAA podcast episode.
See Also
Additional Articles on MTF Wiki
External Resources
- ph Meter and TA kit equipment list with prices and links by Oregon BrewLab.
- Wort and Beer Titration by Kai Troester.
- More information on this procedure is available from the American Society of Brewing Chemists, who publish a similar set of procedures under the name "Total Acidity with Potentiometer".
- Jim Crooks of Firestone Walker presentation about blending sour beers using TA.
- Dave Janssen's discussion of Kara Taylor's CBC talk.
- pH Readings of Commercial Beers, Embrace the Funk Blog, Brandon Jones.
- "Understanding pH and Titratable Acidity in Sour Beer: Tools for Brewers and Enthusiasts Alike" by Stan Hieronymus on the Craft Beer & Brewing Magazine website.
- Amazon source for NaOH.
- MTF discussion regarding Kara Taylor's BA presentation that shows TA for multiple beers, and suggestion for using "Sour Units" as a measurement for beer.
- MTF tips on safety and methods of measuring TA from a research technician.
- "Savoring Acidity: The Quest to Explain Sourness in Beer", by Brian Yaeger in BeerAdvocate magazine.
- "A Guide to Blending Sour Beer With Fruit" by Matt Miller; includes ABV and TA calculators for fruit additions.
- RedCheck Titratable Acidity kit. [17]
- Lallemand Brewing online TA tool and instructions using ASBC methods.
- Blue Owl Brewing uses an altered TA number called "Sour Units" to communicate to customers how acidity their beers are.
Authorship
Originally written by James Howat with major updates by Andy Carter, and with input from Dan Pixley and organic chemist Mike Castagno.
References
- ↑ Wine From the Outside - Easy Wine Chemistry For the Casual Chemist. Monitoring Acids and pH in Winemaking. Mike Miller.
- ↑ The relationship between total acidity, titratable acidity and pH in wine. Roger Boulton. American Journal of Enology and Viticulture. 31(1): 76-80. 1980.
- ↑ How Sour is Your Sour Beer?. OCBeerBlog on Firestone Walker's demonstration of the uses of TA measurements. April 13, 2015.
- ↑ "Relationship between pH and Titratable acid in Cider Apple Juices." The Wittenham Hill Cider Portal (blog). 2011. Retrieved 11/24/2017.
- ↑ 5.0 5.1 Bates, Roger G. Determination of pH: theory and practice. Wiley, 1973.
- ↑ Gump, Barry H. "pH and Titratable Acidity" Presentation. 2014. Retrieved 11/14/2017.
- ↑ Kara Taylor. CBC 2015 Presentation. 2015. Retrieved 11/14/2017.
- ↑ The Wittenham Hill Cider Portal. "Relationship between pH and Titratable acid in Cider Apple Juices". Retrieved 11/14/2017.
- ↑ 9.0 9.1 ASBC Methods of Analysis. Total Acidity. Retrieved 11/8/2017.
- ↑ A novel rate of the reaction between NaOH with CO2 at low temperature in spray dryer. Yadollah Tavan, Seyyed Hossein Hosseini. 2017. DOI: https://doi.org/10.1016/j.petlm.2016.11.006.
- ↑ "Sodium Hydroxide and Carbon Dioxide: Why it is Important to Keep Your Standard NaOH Solutions Capped". Indiana University course material. Retrieved 10/18/2018.
- ↑ Robert Hall. The "Sour" in Sour Beers: Microbiology Sensory Perception and Styles. HomebrewCon 2018 Seminar. 2018.
- ↑ Various MTF members. Milk The Funk thread on what size buret to use for titrations. 12/01/2017.
- ↑ "TN14 - Interconversion of acidity units" Industry Development and Support. Australian Wine Research Institute. Retrieved 09/15/2016.
- ↑ Conversation with Dave Janssen on MTF. 6/23/2015.
- ↑ Dan Pixley. Milk The Funk Facebook group post that summarizes the MBAA Podcast episode 85. 04/29/2018.
- ↑ Kelley Freeman. Milk The Funk Facebook group thread on TA kits. 01/06/2018.