Difference between revisions of "Laboratory Techniques"
m |
m |
||
| Line 1: | Line 1: | ||
| − | This | + | This focuses on home lab and small brewery lab techniques. |
==Equipment== | ==Equipment== | ||
Revision as of 12:34, 18 January 2018
This focuses on home lab and small brewery lab techniques.
Contents
Equipment
General (links to other pages)
Bunsen Burner
Density Meter
Density Meter's are a much more accurate tool for testing things like ethanol content as well as other things that can be useful in a brewery setting.
Dissolved Oxygen Meter
Dissolved oxygen meters play a large role in brewery QC of the final packaged products as well as oxygenation of wort pre-fermentation. Although cheaper DO meters can be used to find the PPM(parts per million) of oxygen in the wort pre-fermentation, more expensive advanced equipment is needed post fermentation as you'll need equipment that is capable of reading much lower levels of DO on a ppb level. When monitoring DO its crucial to be under 100 ppb packaged DO and for hop driven beers its ideal to be under 50 ppb or else you risk oxidation and other off flavors associated with oxygen.
During DO packaging testing, Justin Amaral found that breweries have issues with DO coming directly from their canning/bottling system. The most common factor found in high DO readings was in proper purging of lines when starting up the machines or taking a break as well as mechanical failure.
Orbital Shaker
An orbital shaker is a laboratory device used for mixing substances or maintaining movement of fluids. Maintaining movement of liquids has been shown to help some microorganisms grow. For example, running a shaker at 80 RPM for Brettanomyces starters is an effective way to grow this genra (see Brettanomyces starters) [1]. For a home orbital shaker example, see Example of a Home Lab Orbital Shaker.
PCR/qPCR
https://www.minipcr.com/product/minipcr-dna-discovery-system/
https://www.facebook.com/groups/MilkTheFunk/permalink/1888017211226484/ - Under Joe I's comment
Titratable Acidity Meter
TA meters can be a very useful tool in sour beer brewing. Although its generally been used in the wine world to measure the acidity of wines, it can be used on beer as well. Although pH can give you rough look how acidic something is, it doesn't really give you a accurate outlook on how acidic something is on the palate. Please refer to http://www.milkthefunk.com/wiki/Titratable_Acidity for more info.
UV/VIS Spectrophotometer
A Spectrophotometer can be used for an array of tests. They are commonly used to analyze SRM, IBU, ethanol content and other wort compositions. They can also be used for cell counts as well as other microbiology facets.
UV Plate Cooling Cabinet
Using a UV sterilization cabinet can not only help cool/sterilize freshly poured plates but it can be used to UV sterilize other things as well. The build itself is quite simple. You will have to do some basic wiring.
Ideally you'll want a Laminar flow hood with a UV light in the hood, but this will do for just cooling plates or doing quick UV sterilization.
Parts List -
Light Ballast https://www.amazon.com/gp/product/B00AB32J7S/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 Lamp Mount https://www.amazon.com/gp/product/B0036ZA966/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 4 pin connector https://www.amazon.com/gp/product/B003B92NP2/ref=oh_aui_detailpage_o04_s01?ie=UTF8&psc=1 UV Light https://www.amazon.com/gp/product/B001HB3E2W/ref=oh_aui_detailpage_o06_s00?ie=UTF8&psc=1 Plexiglass https://www.amazon.com/gp/product/B019D0DUDQ/ref=oh_aui_detailpage_o03_s00?ie=UTF8&psc=1
Growth Media
Lactobacillus/Pediococcus
See Rogosa SL Agar
MRS Media
| Chemical | Usage Amount |
|---|---|
| Dextrose | 20 grams |
| Peptone | 10 grams |
| Beef Extract | 8 grams |
| Yeast Extract | 4 grams |
| Sodium Acetate | 5 grams |
| Dipotassium Hydrogen Phosphate | 2 grams |
| Ammonium Citrate | 2 grams |
| Manganous Sulfate Tetrahydrate | 0.05 grams |
| Magnesium Sulfate Heptahydrate | 0.2 grams |
| Distilled/De-ionized Water | Fill to 1000 ML |
NBB Media
NBB media can also be used to help detect/isolate spoilage bacteria. This is a pre-made media by Doehler. More info can be found at NBB Media. Although ASBC suggests this media its most likely favored by them as their recommendations are generally products sold by Siebel. After a quick discussion with Richard Preiss cheaper media can be used to reach the same goals. Using HLP, WLN w/ cycloheximide and tween 80, and MRS w/ cycloheximide and tween 80 you can achieve the same results as using the array of NBB media available.
Saccharomyces
A wide variety of media can be used for Saccharomyces. Bromocresol Green can also be added to these media, as in the commercial WLN formulation. Most Saccharomyces cannot metabolize this dye, causing the colonies to stain green. Chloramphenicol can also be added to eliminate bacterial growth. Although not all of these media are specifically for your average brewers Saccharomyces, most strains should have no issues growing.
YPD Media
| Chemical | Usage Amount |
|---|---|
| Yeast Extract | 10 grams |
| Peptone | 20 grams |
| Dextrose | 20 grams |
| Agar(optional) | 15 grams |
| Distilled Water | Fill to 1000 ML |
MYPG Media
| Chemical | Usage Amount |
|---|---|
| Malt Extract | 3 grams |
| Yeast Extract | 3 grams |
| Peptone | 3 grams |
| Dextrose | 10 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Sabouraud Media
| Chemical | Usage Amount |
|---|---|
| Cycloheximide (Optional) | 10 mg |
| Chloramphenicol (Optional) | 0.5 grams |
| Peptone | 5 grams |
| Dextrose | 20 grams |
| Agar | 15 grams |
| Distilled Water | Fill to 1000 ML |
Freezing Media
| Chemical | Usage Amount |
|---|---|
| Glycerin | 50 grams |
| Ascorbic Acid | 15 grams |
| Liquid YPD/MYPG | Fill to 100 ML |
Beef Broth Media
| Chemical | Usage Amount |
|---|---|
| Beef Broth(No preservatives) | 500 mL |
| NaCI (can substitute non-iodized or sea salt) | 50-200 grams |
| Peptone | 5 grams |
| Dextrose | 10 grams |
| Agar(optional) | 17 grams |
| Distilled Water | Fill to 1000 ML |
Wild Yeast Screening Media
| Chemical | Usage Amount |
|---|---|
| Peptone | 5 grams |
| Yeast Extract | 3 grams |
| Malt Extract | 3 grams |
| Dextrose | 5 grams |
| CuSO4 | 310 mg |
| Distilled Water | Fill to 1000 ML |
LCYM Media
| Chemical | Usage Amount |
|---|---|
| Ammonium chloride | 0.5 grams |
| Dipotassium ortho phosphate | 1.1 grams |
| Cupric sulphate (anhydrous) | 0.55 grams |
| Dextrose | 10 grams |
| DME | 2 grams |
| Peptone | 2 grams |
| Yeast Extract | 4 grams |
| Agar | 17 grams |
| Distilled/De-ionized Water | Fill to 1000 mL |
Brettanomyces
A few different medias can be used to isolate Brettanomyces but DBDM media is commonly used. WLD with additions of cycloheximide can also be used.
DBDM Media Recipe
| Chemical | Usage Amount |
|---|---|
| Yeast nitrogen base (YNB) | 6.5 grams |
| Ethanol | 4% v/v |
| Cycloheximide | 10 mg |
| p-coumaric acid | 100 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 ML |
WLD Media Recipe
| Chemical | Usage Amount |
|---|---|
| Cycloheximide | 4 grams |
| Yeast Extract | 4 grams |
| Pancreatic Digest of Casein(Peptone) | 5 grams |
| Dextrose | 50 grams |
| Monopotassium Phosphate | .55 grams |
| Potassium Chloride | 425 mg |
| Calcium Chloride | 125 mg |
| Magnesium Sulfate | 125 mg |
| Ferric Chloride | 2.5 mg |
| Manganese Sulfate | 2.5 mg |
| Bromocresol Green | 22 mg |
| Agar | 20 grams |
| Distilled Water | Fill to 1000 ML |
Misc/Other
- MTF tips on autoclaving wort extract based media in such a way that prevents precipitation and darkening.
- For techniques on separating yeast strains from themselves and from bacteria, see Yeast/Bacteria Isolation.
- To detect Brettanomyces in a Saccharomyces culture, WLN (or WLN along with DBDM) or even something as cheap as YPD can be used because Saccharomyces will show significant colony growth at 3 days, while Brettanomyces colonies will grow at 5-10 days [2].
Reference on DBDM: https://www.facebook.com/groups/MilkTheFunk/permalink/1805210829507123/?comment_id=1805397166155156&comment_tracking=%7B%22tn%22%3A%22R%22%7D
Storage
- For lactic acid bacteria storage, see Lactobacillus Storage.
- For Brettanomyces storage, see Storing Brettanomyces.
- For S. cerevisiae storage, see Saccharomyces Storage.
Isotonic Sodium Chloride
Isotonic Sodium Chloride is a saline solution which can be used for storage of microbes. Depending on the species, survivability times can range. It should be kept cold just above freezing. Most Saccharomyces strains handle this storage well and can be stored for 2 years like this before needing to re-propagate and re-banking. After one year Lactobacillus and Pediococcus seem to survive moderately well depending on the strain. Brettanomyces however seems to have a harder time maintaining good vitality. Please refer to Justin Amaral's post for information about this.
The solution for Isotonic Sodium Chloride is quite simple. You'll need 9 grams of lab grade Sodium Chloride to 1000 mL of distilled/de-ionized water, autoclaved.
Propagators
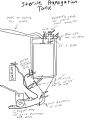
Justin Amaral's 4.5 BBL Propagator
The propagator below has a 14 gallon capacity, allowing a prop up of 4.5 BBL. This same set up can be used on any fermenter but is best used on ones that can hold 5 PSI and up. The seal on the below fermenter can hold around 5 PSI but clamps are also used on the lid to ensure it can hold up to 10 PSI. It essentially acts as a large stir plate while allowing to trickle in either O2, NO2 or CO2 depending on what your propagating. Ideally you'd also use a non magnetic drive pump but its not essential.
Below is the draw up of the build out for this prop up tank. As you can see it uses quick disconnects but you can use tri-clover connects as well. It also uses a diffusion stone that connects into one of the ferrules allowing you to trickle in gases. The picture shows a diffusion stone directly into the fermenter but its since been adapted as shown in the other pictures below to allow the diffusion stone to be behind a valve so its only exposed when needed. It also uses a whirlpool connector that goes into the fermenter creating the whirlpool when the pump is on:
Techniques
(Videos provided by Bryan of Sui Generis blog and Zach Taggart of 42 North Brewing Company.)
Aseptic Technique
Making Agar Plates
- Making WLD plates:
Yeast/Bacteria Isolation
- Mark Trent details his method for isolating Brettanomyces from Saccharomyces from dregs or other mixed cultures.
- See Wild Yeast Isolation.
- See isolating Lactobacillus.
Making Your Own Media
Although media can be bought pre-made, you can also make these media yourself. Media can be either sterilized via an autoclave/pressure cooker or using sterile filtering. Keep in mind it can be difficult however to sterile filter some items such as yeast extract, peptone and brewer's grade DME. Because of this some just autoclave parts of the media while sterile filtering the rest into the sterilized media.
It is crucial you use aseptic technique with your media once it is sterilized to prevent any contamination. If you are storing extra media for later use make sure to remake it every month or 2 if unused.
Yeast Banking
Cell Counting
Gram Staining
Semi-Anaerobic Containers for Incubating Plates
Anerobic chambers can be costly. Here is are two DIY methods. The first achieves under 1% O2, and both methods will also prevent mold growth.
- The plating setup: Use 60mm plates; bigger plates might not fit.
- The anaerobic tools: short wide-mouth jam jar, rubber-lined lid.
- After inoculating, loosely tape the lids in place, just so they don't fall off when placing them in the jar.
- Purge the jar with CO2 for 5-10 seconds with lid just ajar.
- Place 1 or 2 Oxy-Sorb 100-Pack Oxygen Absorber, 100cc into the jar.
- Seal the jar with electrical tape.
- Incubate!
Afterwards, seal the package of Oxy-Sorb with a vacuum sealer. Otherwise they will begin to absorb oxygen [3].
An alternative method using a vacuum sealer and a jar lid adapter:
- The plating setup. Use 60mm plates; bigger plates might not fit.
- The anaerobic tools: short wide-mouth jam jar, lid, vacuum sealer, ball jar attachment.
- After inoculating, loosely tape the lids in place, just so they don't fall off when placing them in the jar.
- Purge the container with CO2 for 5-10 seconds with lid just ajar.
- Place ball jar adapter on jar and vacuum seal container.
- Incubate!
These methods were shared by Bryan of Sui Generis Blog and Zach Taggart of 42 North Brewing Co. See this MTF thread for details.
Shipping Cultures
DeWayne Schaaf recommends the following procedures for shipping cultures with the USA states and territories [4]:
- Use 15ml centrifuge tubes from Cynmar LLC - Wine & Brew. They are great quality and have a very low rate of leakage, especially when combined with electrical tape.
- Place each liquid filled tube into its own snack sized baggie to minimize cross contamination if they do happen to leak.
- Each of these smaller bags will be placed into a quart Ziplock.
- My main shipping container are Uline Poly bubble mailers. I don't tend to use ice packs as I've found them unnecessary when shipping in favorable temps (use ice packs during the summer).
- USPS is my preferred shipping method. Using first class shipping, my packages typically take no more than 3 days to reach somewhere in the USA and cost right around $3, and that includes a tracking number. Shipping rates are roughly the same for any USA state or territory. The 48 continental states, Alaska, Hawaii, Puerto Rico, and Guam all cost roughly the same.
For 5 gallon pitches or cultures that may still be a bit active creating cO2 Soda Preform tubes work very well. These are the same type of tubes White Labs uses for their homebrew pitches. https://www.amazon.com/Soda-Bottle-Preforms-Caps-30/dp/B008MB1QNY/ref=sr_1_1?ie=UTF8&qid=1504524577&sr=8-1&keywords=soda+test+tubes
See also:
Historical
- "Pasteur and the beer of national revenge", by Lars Garshol (how Louis Pasteur proved that microbes were alive in 1857, and other discoveries).
- "Emil Chr. Hansen and the yeast revolution", by Lars Garshol (how yeast was first isolated from other strains in 1883).
See Also
Additional Articles on MTF Wiki
- Microscope
- PH Meter
- Wild Yeast Isolation
- Titratable Acidity
- Saccharomyces
- Brettanomyces
- Pediococcus
- Lactobacillus
External Resources
- BasicBrewing interview with Zack Taggart, lab manager at 42 North Brewing, on how to set up our own homebrew lab to analyze yeast health and count cell population (part 1) and isolating, propagating, and storing yeast and bacteria (part 2).
- "Brewers' Laboratory Handbook: Brewing Without the Blindfold™", by Brewing Science Institute.
References
- ↑ Yakobson, Chad. The Brettanomyces Project. Propagation and Batch Culture Methods. Retrieved 2/18/2015.
- ↑ Richard Preiss, Justin Amaral, and Lance Shaner. Milk The Funk Facebook thread on detecting Brettanomyces in a Saccharomyces culture. 01/18/2018.
- ↑ Bryan of Sui Generis Blog and Caroline (Zach) Taggart on DIY semi-aerobic environment for plating. Milk The Funk Facebook group. 10/05/2017.
- ↑ DeWayne Schaaf. Kveik World Order Facebook page. 04/21/2017. Retrieved 04/24/2017.