Difference between revisions of "Lactobacillus"
(final starter edits for now) |
|||
| Line 59: | Line 59: | ||
===General Advice=== | ===General Advice=== | ||
====Starters and Pitching Rate==== | ====Starters and Pitching Rate==== | ||
| − | In addition to the starter information given in the [[Lactobacillus#Manufacturer_Tips|Manufacturer Tips]] | + | In addition to the starter information given in the [[Lactobacillus#Manufacturer_Tips|Manufacturer Tips]] above, this section includes general advice for ''Lactobacillus'' starters for homebrewers and brewers without access to MRS media. MRS media is much more expensive than the materials required for the method below (DME, apple juice, chalk, and yeast nutrients). See [[Lactobacillus#External_Resources|External Resources]] for additional starter guides. |
| − | Pitching ~0.5-1 liter per ~20 liters of wort (~0.75-1 gallon per barrel) of ''Lactobacillus'' starter is the general guideline. The exact advisable pitching rates of commercial cultures may differ from manufacture to manufacturer. <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1077639885597558/ MTF thread started by Brad Primozic. 5/29/2015.]</ref>. Counting the exact number of cells can be difficult to achieve due to the small size of bacteria, so starter volumes are generally used <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1068323126529234/?comment_id=1068337639861116&offset=0&total_comments=47&comment_tracking=%7B%22tn%22%3A%22R9%22%7D Conversation on MTF with Bryan Heit. 5/6/2015.]</ref>. | + | Pitching ~0.5-1 liter per ~20 liters of wort (~0.75-1 gallon per barrel) of ''Lactobacillus'' starter is the general guideline. The exact advisable pitching rates of commercial cultures may differ from manufacture to manufacturer. <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1077639885597558/ MTF thread started by Brad Primozic. 5/29/2015.]</ref>. Counting the exact number of cells can be difficult to achieve due to the small size of bacteria cells, so starter volumes are generally used instead when talking about ''pitching rates'' for ''Lactobacillus'' <ref>[https://www.facebook.com/groups/MilkTheFunk/permalink/1068323126529234/?comment_id=1068337639861116&offset=0&total_comments=47&comment_tracking=%7B%22tn%22%3A%22R9%22%7D Conversation on MTF with Bryan Heit. 5/6/2015.]</ref>. |
''Starter Procedures:'' | ''Starter Procedures:'' | ||
| − | # Make a 1 liter starter of '''1.040 SG (10°P) | + | # Make a 1 liter starter of '''1.040 SG (10°P) Dried Malt Extract wort with 10% apple juice + 20 grams of chalk (CaCO3) + yeast nutrients''' for growth results that are close to MRS media growth results (as per [https://eurekabrewing.wordpress.com/2015/05/18/evaluate-starter-media-to-propagate-lactobacillus-sp/ Samuel Aeschlimann from Eureka Brewing Blog]). The chalk won't dissolve into solution; don't worry about it. <ref>[https://eurekabrewing.wordpress.com/2015/05/18/evaluate-starter-media-to-propagate-lactobacillus-sp/ Evaluate starter media to propagate Lactobacillus sp., Eureka Brewing Blog, by Samuel Aeschlimann.]</ref>. |
# Best practice is that starters should not be aerated, although there may be an exception to this for ''L. brevis'' <ref name="brevis_aeration"></ref>. | # Best practice is that starters should not be aerated, although there may be an exception to this for ''L. brevis'' <ref name="brevis_aeration"></ref>. | ||
# The starter should be held at the temperature best suited for the culture as shown in the [[Lactobacillus#Culture_Charts|Culture Charts]]. | # The starter should be held at the temperature best suited for the culture as shown in the [[Lactobacillus#Culture_Charts|Culture Charts]]. | ||
# Reference the above [[Lactobacillus#Culture_Charts|Culture Charts]] for how long the starter should be incubated for before pitching. | # Reference the above [[Lactobacillus#Culture_Charts|Culture Charts]] for how long the starter should be incubated for before pitching. | ||
| − | # Pour all of the liquid of the starter into into the wort/beer, leaving the sediment behind | + | # Pour all of the liquid of the starter into into the wort/beer, leaving the sediment behind. The chalk will sediment out, and is not desirable to pitch into the wort/beer. Avoid cold crashing because it can have an adverse effect on the bacteria's health <ref>[http://suigenerisbrewing.blogspot.ca/2015/05/lacto-starters.html Heit, Bryan. Lacto Starters. Sui Generis Blog. Retrieved 6/15/2015.]</ref>. |
See [http://suigenerisbrewing.blogspot.ca/2015/05/lacto-starters.html ''Lacto Starters'', by Bryan Heit of Sui Generis blog] for additional information on ''Lactobacillus'' starters. | See [http://suigenerisbrewing.blogspot.ca/2015/05/lacto-starters.html ''Lacto Starters'', by Bryan Heit of Sui Generis blog] for additional information on ''Lactobacillus'' starters. | ||
Revision as of 14:53, 16 June 2015
Lactobacillus (often referred to as Lacto) is a genus of lactic acid bacteria (LAB) which produces acidity and sour flavors in the form of lactic acid (and sometimes acetic acid) found in lambics, Berliner Weiss, sour brown ales, and gueuze. Lacto can form a pellicle. See Pediococcus, Brettanomyces, Saccharomyces, and Mixed Cultures charts for other commercially available cultures. See the Sour Worting and Mixed Fermentation pages for brewing techniques with Lactobacillus. See the Alternative Bacteria Sources section for culturing Lactobacillus from grains, yogurt, probiotics, and other sources.
Contents
Commercial Lactobacillus Cultures
Culture Charts
| Name | Mfg# | Taxonomy | CO2 Producer (Het/Hom) | Starter Note | Fermentation/Other Notes |
|---|---|---|---|---|---|
| Brewing Science Institute | L. delbrueckii | Lactobacillus delbrueckii | A Lactobacillus bacteria that produces a clean lactic sourness. | ||
| White Labs | WLP677 | L. delbrueckii | Heterofermentative [1] | no stir plate, room temp | Incubate at > 90°F and < 117°F for 5-7 days for greater lactic acid production. |
| White Labs | WLP672 | L. brevis | Heterofermentative [2] | No stir plate, room temp | Produced by The Yeast Bay. More hop tolerant than other Lacto strains, however TYB advises to use wort with less than 10 IBU. Temperature range: 70-95°F; 80% attenuation (this may not reflect actual attenuation of wort in a real brewery; see reference [3]). [4] |
| Wyeast | 5335 | L. buchneri | 1L starter, 1.020 DME sterile wort, no stir plate, no O2, starter at 90°F if possible 5-7 days | Incubate at 90°F for 5-7 days for greater lactic acid production. | |
| Wyeast | 5223-PC | L. brevis | Heterofermentative [2] | no stir plate, room temp is fine | Heterofermentative (produces lactic acid, ethanol and CO2), more hop tolerant. Does well at room temperature. AVAILABLE ONLY FROM JULY THROUGH SEPTEMBER 2014 (Michael Dawson from Wyeast indicated that this culture may return at some point). Jamie Daly indicated on MTF that he got almost no sourness after 24 hours at 100°F (37.8°C). He lowered the temperature to 90°F-95°F (32.2°C-35°C) for 36 hours, and the pH of the wort went down to 3.29. Thus, Jamie recommends 90°F-95°F (32.2°C-35°C) for 60 hours for better souring; avoid warmer temperatures. He also aerated his starter of L. (brevis 2L starter of 1.020 dme) and set it on a stir plate at 95°F [5]. The beer wort was not aerated, and the fermenter was flushed with CO2. These methods need verification. |
| Omega Yeast Labs | OYL-605 | L. brevis, delbrueckii, and plantarum blend | Hetero/Hetero | 1 liter starter at room temperature for 24-48 hours. no stir plate | Quick souring. Pitch into 65°F-90°F. Holding temperature is not required. No longer contains delbruekii [6]. |
| GigaYeast | GB110 | L. delbrueckii?[7] | Heterofermentative | no stir plate, room temp | Incubate at 98°F for 48-72 hours; use low IBU wort. Sometimes referred to as GigaYeast's "Fast Acting Lacto". This strain is hop sensitive [8]. |
| RVA Yeast Labs | RVA 600 | L. rhamnosus GG | Homofermentative | No starter necessary | Homofermentative Lacto strain found in probiotics; sensitive to hops; does well at room temperature. |
| SouthYeast Labs | Lactobacillus 1 | Unknown | Heterofermentative | Source: Spontaneously infected beer (South Carolina). Best suits Light sours, gose, farmhouse saison (medium/high acidity). | |
| SouthYeast Labs | Lactobacillus 2 | Unknown | Homofermentatative | Source: Prickly pear fruit (South Carolina). Best suits strong sours, and lambic (high acidity). |
Manufacturer Tips
Omega Yeast Labs on OYL-605
The following is a statement by Lance Shaner, owner of Omega Yeast Labs:
Lance Shaner: Full disclosure: I own Omega Yeast Labs. Pitching at 120F is a bad idea with this blend. The bug doing most of the work in this blend is Lactobacillus plantarum. The best temp for plantarum is 80-90F. It does not work [as well] over 100F. Also, we regularly make a 1 liter starter with the Lacto blend for faster souring. Simply pitch the contents of the pouch into 1 liter of sterile 1.040 wort and let sit for 24 hours at 70-80F before pitching (no need to stir). Adi Hastings mentioned the imperial stout we just kettle soured. We previously brewed a Berliner using the same method. At 17 hours, pH was at 3.42 and temp was 75F (original pitch temp was 85F). At 40 hours, pH was 3.24, at which time we boiled. Lower pH in the Berliner compared to the stout at 17 hours likely has to do with different buffering capacities in different worts.
Wyeast on 5335
The following is an excerpt with Jess Caudill, Brewer/Microbiologist, at Wyeast Laboratories, Inc. concerning usage of Wyeast 5335 and making a Berliner Weissbier.
- Use 5335.
- If using our 5335, don’t use ANY hops. You can always blend in some IPA or hopped wort after souring takes place if you really need some bitterness or hop flavor/aroma in the beer.
- From one 5335 pack, make a 1L starter with 1.020 DME sterile wort. No O2! Incubate at 90°F if possible for 5-7 days.
- Brew your 5 gallons of wort. Again… no hops. Sterilize the wort. (No need for sour mashes). Cool to 90°F and add 1L 5335 starter. No O2. Try to maintain 90°F for 5-7 days depending on how sour you want the beer.
- After 5-7 days, cool wort to around 68. Pitch with a low pH tolerant strain such as 1007 or 2124. No O2. Ferment for around 1-2 weeks… until you hit terminal.
- Package beer. If bottle conditioning, use 4021 as a bottling strains. Very tolerant to low pH.
RVA Yeast Labs on RVA 600
A great lactic acid bacterial strain that will add a pleasant tangy sourness. RVA 600 is a pure culture of Lactobacillus rhamnosus GG which is found in many commercial probiotic products which have been shown in clinical studies to have many beneficial effects. These are homofermentative (only produces lactic acid, no carbon dioxide or ethanol) and are hop-sensitive. For more pronounces souring add before you add your yeast. You can sour to taste then add a yeast strain to outcompete the bacteria. Again, hop sensitive so easy on them…or dry hop the heck out of it! You may also want to experiment with blending sour low hop beer with an ale strain beer. [9]
... the amount of bacteria in our homebrew units should eliminate the use of a starter. Pitching on the warm side will speed up the souring but RVA 600, a pure culture of Lactobacillus rhamnosus GG, the first commercially available probiotic propagated for use in brewing, does just fine at room temp. We had originally developed RVA 600 as a mix but fell in love with the pure strain.[10]
SouthYeast Labs on Lactobacillus 1 and 2
The L2 strain grows best at 86°F-100°F (30°C-37.7°C), and does not work well over 100°F. Keep IBU's low to none. L1 will likely be discontinued due to high amounts of acetic acid production [11].
White Labs on WLP672
"It is intended for secondary, so you only need to do a starter if you are doing a primary fermentation with it. DME would be the best substrate... Since its a Lacto species, you don't really want to aerate it. A slow stir-plate would be good, to keep it moving, but not much more than that." - Sarah Neel, White Labs, Sales and Customer Service (quoted with permission).
General Advice
Starters and Pitching Rate
In addition to the starter information given in the Manufacturer Tips above, this section includes general advice for Lactobacillus starters for homebrewers and brewers without access to MRS media. MRS media is much more expensive than the materials required for the method below (DME, apple juice, chalk, and yeast nutrients). See External Resources for additional starter guides.
Pitching ~0.5-1 liter per ~20 liters of wort (~0.75-1 gallon per barrel) of Lactobacillus starter is the general guideline. The exact advisable pitching rates of commercial cultures may differ from manufacture to manufacturer. [12]. Counting the exact number of cells can be difficult to achieve due to the small size of bacteria cells, so starter volumes are generally used instead when talking about pitching rates for Lactobacillus [13].
Starter Procedures:
- Make a 1 liter starter of 1.040 SG (10°P) Dried Malt Extract wort with 10% apple juice + 20 grams of chalk (CaCO3) + yeast nutrients for growth results that are close to MRS media growth results (as per Samuel Aeschlimann from Eureka Brewing Blog). The chalk won't dissolve into solution; don't worry about it. [14].
- Best practice is that starters should not be aerated, although there may be an exception to this for L. brevis [5].
- The starter should be held at the temperature best suited for the culture as shown in the Culture Charts.
- Reference the above Culture Charts for how long the starter should be incubated for before pitching.
- Pour all of the liquid of the starter into into the wort/beer, leaving the sediment behind. The chalk will sediment out, and is not desirable to pitch into the wort/beer. Avoid cold crashing because it can have an adverse effect on the bacteria's health [15].
See Lacto Starters, by Bryan Heit of Sui Generis blog for additional information on Lactobacillus starters.
Cell Growth
"I typically grow it by itself anaerobically in MRS media. Seems to work very well and results in good growth. I've personally had the best success with MRS media and in an anaerobic environment, though I know some lactobacillus strains grow aerobically just fine. The problem with growing lactic acid bacteria is the acid they produce will eventually inhibit their own growth. MRS contains a buffer to help combat the drop in pH as a result of LAB metabolism, which keeps the pH around 6-6.5 (I think) for optimal growth. I usually grow them at 35 C, but sometimes incubator space is at a premium (like right now) and I just spin it on the benchtop!" [16]. ~ Nick Impellitteri from The Yeast Bay on general Lactobacillus cell growth
Hop Tolerance
"There is a fair bit of research into hop tolerance out there; its not a simple topic as a number of factors come into play to produce hop tolerance. To make things even more complicated, hop tolerance is an inducable trait in many lacto species - meaning that a seemingly susceptible strain can become resistant by culturing in ever-increasing doses, and a seemingly resistant strain can become susceptible after a generation or four in a hop-free media. ~ Bryan Heit of Sui Generis Brewing blog on Lactobacillus and Hop Tolerance
Edit: I tried to add (but facebooks aversion to white space almost defeated me) that I've been trying to generate a permanently high-alpha acid resistant lacto strain for a few months now. I've been culturing L. brevis in escalating IBU wort (starting at 10, currently at 25). Every 4th generation (1 generation = a subculture of a stationary-phase lacto culture, not as in # cell divisions) I pass it through 2 generations of an IBU-free media to try and select for strains which maintain this resistance. This seems to have worked upto ~18 IBU, but past that point the resistance appears to remain inducable. I'm hoping a few more generations will provide me with a permanently tolerant strain.
There are some other option; I've purified (but didn't keep - doh) some pretty resistant strains from grain by by making plates where you half-fill a plate, on an angle, with a high-IBU wort, and then overlay that with a no-IBU wort. This gives you a gradient plate, with low-IBUs on the end where the hopped-wort layer is thinnest and high IBUs where it is thickest. Some of those strains were resistant to over 30IBU, but being early in my yeast farming days I didn't bother keeping those.
I'm hoping to have a blog post on that sometime in the next few months, but life is nuts right now so I offer no guarantee." [17]
Storage
Regarding dry Lactobacillus, such as probiotics or Dry Yeast for Sour Ales BlackManYeast products: "Yes, refrigerate them. In the lab course I run we compare probiotics stored at room temp versus in the fridge - viable bacterial numbers dip off ~80x faster in non-refrigerated samples."
In regards to liquid cultures and storage: "I'm not sure about the liquid cultures - I freeze mine at -80°C (lab freezer), which (stores) indefinitely. As a rule refrigerated liquid cultures should last longer... but there is anecdotal evidence (for some species) of poorer survival of refrigerated versus room temp in liquid cultures. IMO, stable temps are likely more important for non-frozen stocks than hitting an 'ideal' temperature." [18] ~ Bryan Heit of Sui Generis Brewing blog.
Commercially available Lactobacillus strains and their pH change over time
All data provided by Matt Humbard
pH change at 86°F
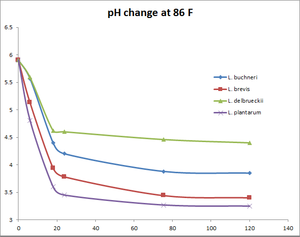
| Time (hours) | Wyeast Lactobacillus buchneri | White Labs Lactobacillus brevis | White Labs Lactobacillus delbrueckii | Omega Lactobacillus plantarum |
|---|---|---|---|---|
| 0 | 5.9 | 5.9 | 5.9 | 5.9 |
| 6 | 5.57 | 5.13 | 5.59 | 4.81 |
| 18 | 4.4 | 3.94 | 4.62 | 3.6 |
| 24 | 4.2 | 3.78 | 4.6 | 3.45 |
| 75.5 | 3.88 | 3.44 | 4.46 | 3.27 |
| 120 | 3.85 | 3.4 | 4.4 | 3.25 |
pH change at 98°F
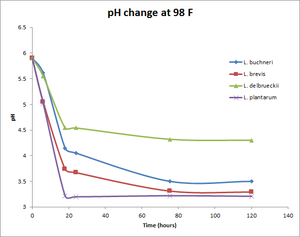
| Time (hours) | Wyeast Lactobacillus buchneri | White Labs Lactobacillus brevis | White Labs Lactobacillus delbrueckii | Omega Lactobacillus plantarum |
|---|---|---|---|---|
| 0 | 5.9 | 5.9 | 5.9 | 5.9 |
| 6 | 5.61 | 5.05 | 5.55 | 5.02 |
| 18 | 4.15 | 3.74 | 4.54 | 3.22 |
| 24 | 4.05 | 3.67 | 4.54 | 3.2 |
| 75.5 | 3.5 | 3.31 | 4.32 | 3.22 |
| 120 | 3.5 | 3.29 | 4.3 | 3.21 |
pH change at 102°F
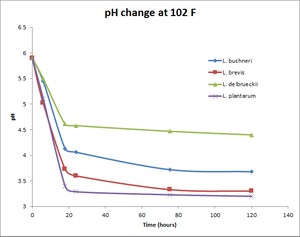
| Time (hours) | Wyeast Lactobacillus buchneri | White Labs Lactobacillus brevis | White Labs Lactobacillus delbrueckii | Omega Lactobacillus plantarum |
|---|---|---|---|---|
| 0 | 5.9 | 5.9 | 5.9 | 5.9 |
| 6 | 5.45 | 5.02 | 5.5 | 5.11 |
| 18 | 4.13 | 3.73 | 4.61 | 3.4 |
| 24 | 4.06 | 3.6 | 4.58 | 3.29 |
| 75.5 | 3.72 | 3.33 | 4.47 | 3.23 |
| 120 | 3.68 | 3.3 | 4.4 | 3.2 |
pH change at 108°F
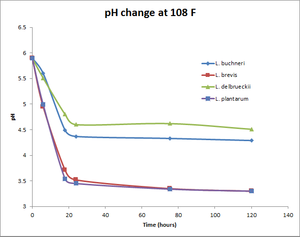
| Time (hours) | Wyeast Lactobacillus buchneri | White Labs Lactobacillus brevis | White Labs Lactobacillus delbrueckii | Omega Lactobacillus plantarum |
|---|---|---|---|---|
| 0 | 5.9 | 5.9 | 5.9 | 5.9 |
| 6 | 5.6 | 4.95 | 5.51 | 4.99 |
| 18 | 4.49 | 3.71 | 4.8 | 3.54 |
| 24 | 4.37 | 3.52 | 4.6 | 3.45 |
| 75.5 | 4.33 | 3.35 | 4.62 | 3.34 |
| 120 | 4.29 | 3.3 | 4.51 | 3.3 |
Metabolism
- Editor's note: the following section was reviewed for accuracy by MTF members Bryan Heit,
Matt Humbard, Lance Shaner, and Richard Preiss.
Types of Metabolism
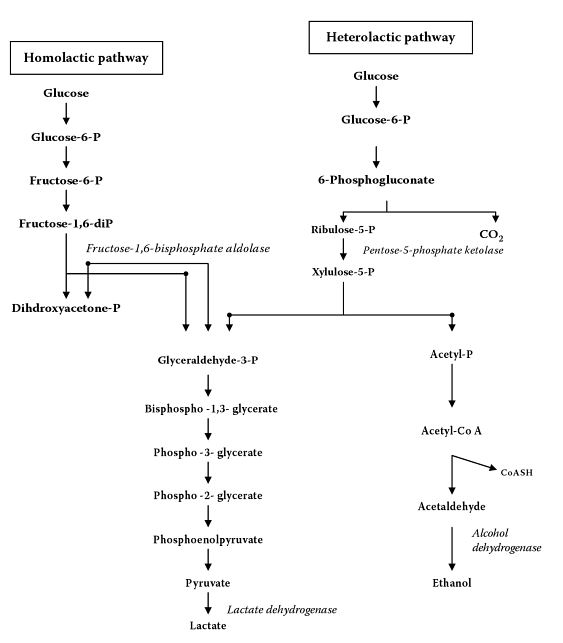
All metabolism by Lactobacillus, including growth, will require sugar to be consumed and lactate (lactic acid) to be produced. Two categories of metabolism exist, homolactic and heterolactic. In summary, homolactic fermentation produces only lactic acid, while heterolactic fermentation produce lactic acid, CO2, and ethanol/acetic acid [20].
Homolactic
Homolactic metabolism is described as the cell catabolizing one molecule of glucose to yield two molecules of pyruvate, which is then further reduced to two molecules of lactate (lactic acid). Homolactic fermentation only allows the fermentation of hexoses (glucose, mannose, etc.). Homolactic metabolism follows the Embden-Meyerhof-Parnas pathway [19].
Heterolactic
Heterolactic metabolism is described as the cell catabolizing one molecule of glucose into one molecule of CO2, one molecule of glyceraldehyde phosphate, and one molecule of acetyl phosphate. The molecule of glyceraldehyde phosphate is reduced to one molecule of lactate, and the acetyl phosphate is reduced to one molecule of ethanol (or one molecule of acetic acid instead of ethanol, depending on its growing environment [21]). Heterolactic fermentation allows the fermentation of hexoses and pentoses [22]. Heterolactic fermentation follows the pentose phosphate pathway, or also called the phosphogluconate pathway [19].
Categories of Lactobacillus
There are three categories of Lactobacillus based on the type of fermentation they are capable of (homolactic, heterolactic, or both).
- Obligatory homofermentative Lactobacillus only perform homolactic fermentation, and thus only produce lactic acid [19].
- Obligatory heterofermentative Lactobacillus only perform heterolactic fermentation, and thus produce lactic acid, CO2, and ethanol (or sometimes acetic acid instead of ethanol) [19].
- Facultatively heterofermentative Lactobacillus generally are homolactic when there is an abundance of carbohydrates, but can also perform heterolactic fermentation when carbohydrates are not abundant [19].
Other factors can determine if a facultative heterofermentative species uses homolactic or heterolactic fermentation. For example, L. plantarum, which is a facultatively heterofermentative species, is homolactic without the presence of oxygen. In the presence of oxygen, however, it performs heterolactic fermentation, and produces acetic acid [23][24].
100% Lactobacillus Fermentation
The amount of CO2 produced is very small in heterofermentative species. Lance Shaner of Omega Yeast Labs noted that although L. brevis is classified as obligatory heterofermentative, the human eye cannot detect any CO2 production in the Omega Yeast Lactobacillus blend (OYL-605). Lance still needs to test this blend to see if it produces any CO2 at all. It is clear though that any type of Lactobacillus, regardless of whether it is heterofermentative or homofermentative, cannot produce a krausen. Krausens are sometimes seen with the use of commercially available Lactobacillus cultures. If a krausen develops in wort when it is the only culture that is pitched, this is indicative of cross contamination of Saccharomyces or Brettanomyces in either the wort, or the Lactobacillus culture itself [25]. In addition to this, heterolactic fermentation by Lactobacillus can only produce 10-20% of the ethanol that Saccharomyces can produce [26], therefore a high level of attenuation cannot be achieved by Lactobacillus and is again a sign of cross contamination by yeast.
| Obligatory Homofermentative [27] | Obligatory Heterofermentative | Facultatively Heterofermentative |
|---|---|---|
| L. acidophilus | L. brevis | L. casei |
| L. delbruekii | L. buchneri | L. curvatus |
| L. helveticas | L.fermentum | L. plantarum |
| L. salivarius | L. reuteri | L. sakei |
| L. pontis |
Sugar Utilization and Secondary Metabolites
For a chart and in depth discussion on what types of sugars are fermentable by different species of Lactobaccilus, as well as charts on secondary metabolites, see Matt Humbard's Physiology of Flavors in Beer – Lactobacillus Species blog article.
In summary:
- Different species of Lactobacillus are capable of fermenting different types of sugars, including sugars that Saccharomyces may not be able to ferment.
- All types of Lactobacillus produce different levels of secondary metabolites (compounds that are not required for the organism to live [28]) in addition to the primary metabolites discussed above. These include acetaldehyde, diacetyl, fusel alcohols, and many more compounds (see Matt's article for more details).
See Also
Additional Articles on MTF Wiki
- Alternative Bacteria Sources
- Mixed Cultures
- Mixed Fermentation
- Sour Worting
- Scientific Publications
- Pediococcus
External Resources
- Physiology of Flavors in Beer – Lactobacillus Species - Matt Humbard's overview of different species of Lactobacillus physiology, discussion on homoefermentative vs heterofermentative physiology, which species can ferment different types of sugars, and secondary metabolites. Extensive data points included.
- Lactobacillus Starter Guide by Derek Springer. - Information about starters for both pure strains, as well as culturing from grains.
- Evaluate starter media to propagate Lactobacillus sp., Eureka Brewing Blog, by Samuel Aeschlimann. - This experiment showed that growing Lactobacillus in 10°P DME, 10% apple juice + CaCO3 (20 g L-1) + yeast nutrients lead to the best growth results, and close to expensive MRS media growth results.
- Lacto Starters, by Bryan Heit of Sui Generis blog - Step by step guide to making a starter for Lactobacillus.
References
- ↑ Commercial Brettanomyces, Lactobacillus, and Pediococcus Descriptions. The Mad Fermentationist Blog. Michael Tonsmeire. Retrieved 3/4/2015.
- ↑ 2.0 2.1 Conversation with Nick Impellitteri from The Yeast Bay on the MTF Facebook Group. 3/4/2015.
- ↑ Conversation with Michael Soo and Nick Impellitteri on the Milk The Funk Facebook Group. 3/5/2015.
- ↑ The Yeast Bay website. Retrieved 3/2/2015.
- ↑ 5.0 5.1 Growth Response of Lactobacillus brevis to Aeration and Organic Catalysts. J. R. Stamer and B. O. Stoyla. Appl Microbiol. Sep 1967; 15(5): 1025–1030.
- ↑ Conversation with Raymond Wagner of Oso Brewing Co on Milk The Funk. 4/30/2015.
- ↑ From Gigayeast, Inc. on Facebook, 12/3/2014: "Appears to be L. delbrueckii."
- ↑ Conversation with Steve Smith of GigaYeast on MTF. 05/08/2015.
- ↑ From RVA Yeast Lab's Website
- ↑ Discussion on Milk The Funk Facebook group with Malachy McKenna
- ↑ Conversation with David Thorton on MTF Facebook Group. 2/27/2015.
- ↑ MTF thread started by Brad Primozic. 5/29/2015.
- ↑ Conversation on MTF with Bryan Heit. 5/6/2015.
- ↑ Evaluate starter media to propagate Lactobacillus sp., Eureka Brewing Blog, by Samuel Aeschlimann.
- ↑ Heit, Bryan. Lacto Starters. Sui Generis Blog. Retrieved 6/15/2015.
- ↑ Conversation with Nick Impellitteri on Milk The Funk Facebook group. 3/5/2015.
- ↑ Conversation with Bryan Heit on Milk The Funk. 01/19/2015.
- ↑ Conversation with Bryan Heit on Milk The Funk. 05/04/2015.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Fermentation: Effects on Food Properties. Bhavbhuti M. Mehta, Afaf Kamal-Eldin, Robert Z. Iwanski. CRC Press, Apr 12, 2012. Pg 76,77.
- ↑ Todar's Online Texbook of Bacteriology. Kenneth Todar, PhD. Retrieved 05/06/2015.
- ↑ Lactic Acid Bacteria. Raunak Shrestha. Retrieved 6/7/2015.
- ↑ Handbook of Dough Fermentations. Karel Kulp, Klaus Lorenz. CRC Press, May 20, 2003. Pg 33.
- ↑ Lactobacillus plantarum and its biological implications. Microbe Wiki. Retrieved 6/7/2015.
- ↑ Conversation with Lance Shaner about L. plantarum on MTF. 6/7/2015.
- ↑ Discussion with Lance Shaner on MTF. 6/7/2015.
- ↑ Humbard, Matt. Physiology of Flavors in Beer – Lactobacillus Species. Retrieved 6/14/2015.
- ↑ Lactic Acid Bacteria: Microbiological and Functional Aspects, Fourth Edition. Sampo Lahtinen, Arthur C. Ouwehand, Seppo Salminen, Atte von Wright. CRC Press, Dec 13, 2011. Pg 80.
- ↑ Secondary Metabolite. Wikipedia. Retrieved 6/9/2015.