Difference between revisions of "Grain"
(→Amaranth) |
(→Alternative Grains) |
||
| Line 15: | Line 15: | ||
* [https://www.facebook.com/groups/MilkTheFunk/permalink/1655836997777841/?match=YnVja3doZWF0 MTF thread on malted vs raw buckwheat.] | * [https://www.facebook.com/groups/MilkTheFunk/permalink/1655836997777841/?match=YnVja3doZWF0 MTF thread on malted vs raw buckwheat.] | ||
* [https://mdpi-res.com/d_attachment/plants/plants-11-00756/article_deploy/plants-11-00756.pdf Buckwheat and Amaranth as Raw Materials for Brewing, a Review.] | * [https://mdpi-res.com/d_attachment/plants/plants-11-00756/article_deploy/plants-11-00756.pdf Buckwheat and Amaranth as Raw Materials for Brewing, a Review.] | ||
| + | |||
| + | ===Kernza=== | ||
| + | * [https://www.masterbrewerspodcast.com/304 MBAA Podcast Episode #304; Kernza.] | ||
===Millet=== | ===Millet=== | ||
Revision as of 02:01, 1 June 2024
(This page is in progress)
For a general overview of grain in brewing, see Homebrew Talk Wiki page on Grain.
Contents
Alternative Grains
Acidulated Malt
Acidulated malt (or acid malt) is often used in brewing to lower the mash pH when chemical acids (phosphoric or lactic acids) are not allowed or not desired. Acid malt is generally produced by one of three different methods. The first method to create acid malt is to hold germinating barley under anaerobic conditions for 24 hours or more to allow the lactic acid bacteria naturally found on the barley to produce lactic acid. The acidified barley is then kilned. A second method is to spray malt with Lactobacillus and then hold the malt at 50°C for 24-36 hours before kilning. The third method is to steep kilned malt in hot water at 45-50°C until the lactic acid bacteria naturally found on the malt (or sprayed onto it) produces around 1% lactic acid. The wet, acidic malt is then dried which concentrates the lactic acid to between 2 and 4% [1]. Due to the uncontrolled nature of lactic acid bacteria used in the production of acid malt, lactic acid levels can vary from maltster to maltster and potentially batch to batch.
Amaranth
- Vanderbilt University's Center for Latin American Studies and Tennessee State University along with Von Seitz Theoreticales presentation on amaranth in beer.
- Buckwheat and Amaranth as Raw Materials for Brewing, a Review.
- A Review of Recent Studies on the Antioxidant Activities of a Third-Millennium Food: Amaranthus spp.
Buckwheat
- MTF thread on malted vs raw buckwheat.
- Buckwheat and Amaranth as Raw Materials for Brewing, a Review.
Kernza
Millet
Suppliers:
Quinoa
- Quinoa (quinua): a pseudocereal, it is advised to wash the plant and lightly scrub it to remove the "grainy/funky" character, and use a cereal mash. It is difficult to mill due to the small size of the seed [2].
- Scott Janish blog article on brewing sour beer with quinoa.
Rice
- MTF threads on wild rice.
- MTF thread on using sushi rice and "Sacch Trois" to make a DIPA with sake-like characteristics.
Rye
Spelt
- MTF tips on where to buy malted spelt, which has been popularized by The Rare Barrel.
- Tips on where to buy malted spelt.
Misc
Gluten-Free/Reduced
Many of the grains listed above are gluten-free and are used in gluten-free brewing. See the links below for more information on gluten-free brewing in general.
Some sour beers have been reported by Taubman et al. to be gluten reduced, but the beer brands were not listed in the study and the exact mechanism by which they became gluten reduced is not clear. Regardless, their work showed that certain strains of Lactobacillus brevis, L. curvatus, and L. plantarum and a strain of Pediococcus pentosaceus were able to produce gluten reduced beers but only when they were fermented with just these strains for 35-50 days [3][4]. Unfortunately, fermenting with just lactic acid bacteria for this long produces unacceptable flavors and is not a practical way of producing sour beer (see Wort Souring).
See also:
- Eckert Malting & Brewing - a good source for gluten-free malts.
- Grouse Malt House; dedicated to gluten-free malting.
- Colorado Malting Company has a separate facility for gluten-free malting. [5]
- Zero Tolerance Gluten-Free Home Brew Club
- Gluten free sour brewing with Joe Morris on MTF.
Unmalted Barley
Unmalted barley can contain higher levels of manganese ions, which can lead to oxidation as the resulting beer ages. Not all unmalted grain has higher levels of manganese ions; one study found that unmalted spelt does not contain elevated levels of this metal ion [6].
Smoked Grains
Smoked grains may contain small levels of nitrosamines, a known carcinogen [7][8].
Enzymes, Extract Potential, and Nutrients
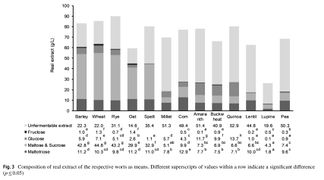
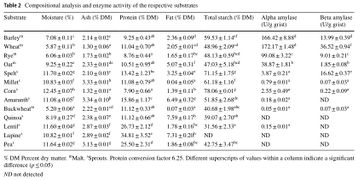
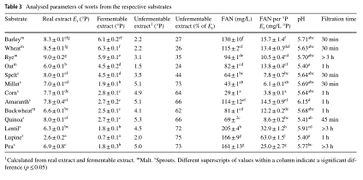
The following figures were sourced from "On the suitability of alternative cereals, pseudocereals and pulses in the production of alcohol-reduced beers by non-conventional yeasts." Konstantin Bellut, Maximilian Michel, Martin Zarnkow, Mathias Hutzler, Fritz Jacob, Kieran M. Lynch, Elke K. Arendt. Eur Food Res Technol (2019). DOI: https://doi.org/10.1007/s00217-019-03372-3. The grains tested were; Bestmalz Pilsen malt (grain), Muntons wheat malt, Betsmalz rye malt, Muntons oat malt, Ziegler spelt sprouts flour, Ziegler brown millet sprouts flour, Keimkraft corn sprouts, Ziegler amaranth sprouts, Mälzerei buckwheat malt, Ziegler quinoa sprouts, Keimkraft lentil sprouts, Keimkraft lupine sprouts, and Keimkraft pea sprouts.
For the millet, quinoa, spelt, buckwheat, amaranth, and corn, starch amylase and protease enzymes were added to liquefy the starch. For the lentil, lupine, and pea, only protease was added. More fermentable sugars were not produced for these grains, but more fermentable sugar such as glucose, maltose, and maltotriose, could be produced with the addition of alpha and beta-amylase from barley malt. The following figures from Bellut et al. (2019) are re-posted here with the permission of Konstantin Bellut [9]:
Composition of real extract from different grain types [10].
Enymes, starch, protein, and fats [10].
Real extract, fermentable extract, and FAN [10].
Amino acid content [10].
Microbial Populations on Barley
Malting Process
Microbial communities found on the outside surface of barley are very diverse and change significantly throughout the malting process, with more diversity occurring further along as the malting process continues. On barley in fields, Gram-negative bacteria (especially Erwinia herbicola) are abundant. Climate is said to have the most impact on which species grow in barley fields. For example Fusarium species are encouraged by high humidity, and are associated with mycotoxins and gushing problems in beer. During dry storage of barley, spore forming bacteria tend to survive. Xerophilic fungi (fungi that doesn't need much water) also survive on dry barley during storage [11].
Microbial communities on the husks of barley change quite a bit during the malting process. It is thought that the factors affecting this are the initial microbe populations, interactions between species, the variances in the malted barley characteristics/processes, and additives. Different malting houses are likely to form "in-house" microflora populations. The first step in malting, which is steeping, sees the first large change in microbial populations. The steeping process favors lactic acid bacteria (LAB), which are seen only in very small numbers before steeping. Particularly these are Leuconostoc species. In the case of yeasts, Basidiomycota fungi grow, as well as Fusarium [11].
The second step of malting, which is germination, sees starches converted to sugars, which causes microbial populations to increase once again. Where Leuconostoc species of bacteria were dominate during steeping, the germination process is where Lactobacillus species begin to dominate the microbial community. A greater diversity of species also occurs during this step in malting. As far as fungi, Ascomycetous yeasts begin to dominate, where as Alternaria and Cladosporium decline [11].
During kilning, microbial populations are reduced by a factor of 10-100, but are still higher than that of field barley. The high degree of variability is dependent on the kilning temperature and procedure. Heat resistant microbes, perhaps by forming biofilms, can survive the hot temperatures. These include molds such as Rhizopus and Mucor species [11].
Malted Barley
Most studies on microbe populations on malt have been done using traditional lab media such as agar plates, which are thought to not be as effective at analyzing the overall population as recent DNA sequencing approaches, such as 454 amplicon pyrosequencing. A recent study took this approach to measuring bacterial diversity on barley and malt (fungi was not analysed). This study looked at two malting houses during two different years (2010 and 2011). It found that on malt, the largest communities of bacteria were found to include Enterobacter, Sphingobacterium, Weissella, Lactobacillus, Lactococcus, Streptococcus, Acinetobacter, and Strenotrophomonas, Leuconostoc, Pseudomonas, Wautersiella, Cryseobacterium, Curtobacterium, and Propionobacterium [12].
Enterobacter spp specifically have been identified as beer spoilage agents, which produce high amounts of 2,3-Butanediol (buttery taste [13]), as well as acetoin (also buttery taste [14]), lactic acid, acetic acid, succinic acid, high alcohols such as n-propanol, iso-butanol, D-amyl-alcohol, isoamyl alcohol, and sulfur compounds such as dimethyl sulphide (DMS), all under both aerobic and semi-aerobic conditions [15][16]. Enterobacter is not inhibited by hops, but is inhibited by ethanol and a pH below 4.5. Acinetobacter has also been shown to produce DMS in wort, however they are strictly aerobic and encountered in much smaller amounts than Enterobacter in brewing [17].
Interestingly, the study also found that the dominate genera of microbes was different between the two years sampled. In 2010, there were more Firmicutes (which includes LAB) and more Actinobacteria, and less Bacteroidetes. In 2011, the reverse was found with fewer Firmicutes and Actinobacteria, and more Bacteroidetes [18] (~15 mins in).
The study examined LAB populations specifically due to their potential to help with malt stability and quality [19]. Streptococcus was the most abundant genus of bacteria in all samples. In 2010, Lactobacillus was more abundant than it was in 2011. Lactococcus and Weisella were more abundant in 2011 than in 2010. Maltster 1 had a greater abundance of both Lactococcus and Weisella in 2011 over Maltster 2. In 2010, Lactobacillus was more abundant for Maltster 2 than it was for Maltster 1. This showed that microbial populations differ not only between malt houses, but also between barley harvest years [18] (~17 mins in). Sometimes culturing from malted grains turns out bad perhaps not because of the brewer, but because of the dominate cultures on the malted grains.
Another study focused on microbial populations in an American coolship brewery detected only Pediococcus on grain samples, but the lack of detection of other microbes was attributed to the methods used [20].
- See this study for a list of microbes that were found on malt using DNA methods (this study found a very small population of Clostridium jejuense on one malt sample).
- See Table 2 of this study for a list of microbes that were found using traditional plating methods.
- See this video presentation by Bart Lievens at the 2015 Belgian Brewers Conference:
Mash and Wort
During mashing, the population of microbes diminishes greatly due to near pasteurization temperatures. However, thermotolerant microbes do survive. These are usually homofermentative LAB [16]. These microbes can have both positive and negative impact on wort production. Mash acidification by thermotolerant L. amylovorus has shown to improve enzymatic conversion of starches to sugars, increased extract and fermentability, increased TSN and FAN, and even improved head retention and increased shelf stability [21]. Bacterial growth during mashing can also have a negative impact. For example, the thermotolerant Gram-positive and facultative anaerobe Bacillus coagulans can cause the mash to go sour and has been shown to form nitrosamines in wort that was supplemented with nitrate. B. coagulans forms nitrosamines without oxygen between the temperatures of 86°F/30°C and 154°F/68°C, and can withstand a pH of 4.0 or higher [22]. Clostridium, which probably does not necessarily originate from the malt itself (so far studies have shown very little to no Clostridium is present on malt), can create butyric acid off flavors during the mash or during kettle souring [23]. High bacterial growth can cause lautering issues, probably due to the production of dextrans by the bacteria [16].
Malt Inoculated Wort
In 2016 and 2017, Dr. Matt Bochman of Indiana University and Jeff Young of Blue Owl Brewing conducted an experiment to map the microbiome of wort that was inoculated with harvest years and varieties of malted barley. Using DNA sequencing, they analyzed the microbes present in wort that was inoculated with crushed malted barley and incubated at 110°F for 0 hours, 24 hours, and 48 hours. The 7 malted grains that used to inoculate the wort were:
- 2015 Breiss Merit 57
- 2016 Breiss Merit 57
- 2015 Patagonia Sebastien
- 2015 Briess Copeland
- 2016 Blacklands Endeavor
- 2016 Breiss Synergy
- 2016 Weyermann Barke
The grain samples before inoculation hosted a large variety of microbes, but immediately after inoculating the malted grains into 110°F wort, all of the grain samples were dominated by Weissela cibaria (ranging from 92-99% across all samples), a Gram-positive lactic acid bacteria that is in the same order but a different family as Lactobacillus [24]. All but one of the grain samples also had a much smaller but not insignificant population of Salmonella bongori (1-4% across all samples), which has been associated with non-lethal food poisoning [25]. There were also trace populations of other bacteria such as Enterobacter spp., Pantoea spp., Erwinia spp., and Lactococcus lactis in most of the samples. There was a significant L. lactics population in the 2016 Weyermann Barke, as well as Enterobacter aerogenes [26].
After 24 hours of continued 110°F incubation in the wort, all but 2 samples were almost completely dominated by Weisella cibaria (98-99% across all samples). The 2016 Weyermann Barke also had a 5% population of Lactococcus lactis. The 2016 Breiss Merit 57 was significantly different than the other grain samples, and was the only sample with a very large population of several species of Lactobacillus (38% Lactobacillus reuteri, 23% Weissla cibaria, 15% Lactobacillus delbruekii, 12% Pedioccocus pentosaceus, 8% Lactobacillus fermentum, and 3% Lactobacillus helveticus). This data indicates that Lactobacillus is generally not the dominant organism when inoculating with grain samples at this temperature, despite popular belief. It was the thought of the authors that Lactobacillus species might dominate at lower or higher temperatures, but more studies are needed to show this. In addition, only a very small population is required to begin with on samples that do, and once inoculated into wort at 110°F, their population will increase significantly [26].
After 48 hours, Weissella cibaria continued to dominate all of the grain samples except the 2016 Breiss Merit 57 (note that the 2015 harvest of Breiss Merit 57 was dominated by Weisella cibaria, indicating that the barley variety is not the determining factor of which microbes might be dominant, but that the harvest year is more important). Two samples, the 2015 Breiss Merit 57 and the 2016 Blacklands Endeavor, also saw an increase in the population of Shiggela sonnei (2.5%). The Salmonella bongori was still present in samples after 48 hours, but decreased to less than 1% [26].
Some of the organic acids and alcohols were also measured in this experiment. Most of the samples created fairly high and similar levels of lactic acid, followed by a smaller amount of acetic acid, and then followed by trace amounts of citric, pyruvic, malic, and succinic acids. The 2016 Breiss Synergy had much more lactic acid, acetic acid, and noticeably more succinic acid the all the other samples. It also had considerably more ethanol produced, indicating that a heat tolerant wild yeast may have fermented this sample [26]. Butyric Acid and Isovaleric Acid were not measured due to the difficulty in measuring these acids at the lab that was used [27].
The experiment concluded that using grain to inoculate wort could potentially be done in a controlled manner, producing predictable and consistent results. It was stated that more studies need to be conducted to explore the variability in brewing process such as temperature and incubation times. For example, while the Weisella cibaria dominated at 110°F, it is reported that it doesn't tolerate temperatures above 113°F, and Lactobacillus might dominate at temperatures around 90-95°F or maybe at temperatures above 113°F [26][28].
Various concerns on possible cross contamination associated with the methodology of the referenced experiment have been discussed with the authors on this MTF thread.
See also:
- "Mapping the sour beer microbiome" by Matthew Bochman and Jeff Young.
- ASBC presentation by Jeff Young.
- Jeff Young's microscopy testing souring wort with grains at different temperatures.
Spent Grain
https://onlinelibrary.wiley.com/doi/pdf/10.1111/jam.13778
Miscellaneous
ASBC Hot Steep Malt Sensory Method
The American Society of Brewing Chemists created a method for malt sensory analysis that is more accurate for evaluating malt flavors than performing a congress mash or simply chewing malted grain. The method is simple and can be done at home [29]:
- Heat distilled/RO water to 65°C (149°F).
- Measure 400 grams of this heated water.
- Mix in 50 grams of milled malt into the 400 mL of 65°C water.
- Put the mixture into a thermos and shake for 20 seconds.
- Let the thermos sit for 15 minutes.
- Shake the thermos a second time for 20 seconds.
- Pour the mixture through a coffee filter; leave for 30-45 mins to fully extract.
- Cool the wort to room temperature and serve no later than 4 hours from the preparation time.
- Notes:
- Evaluate base malts as 100% of the sample, specialty malts with 50% of the specialty malt and 50% of a base malt, and dark-roasted malts with 15% of the dark-roasted malt and 75% of a base malt.
- If different samples are being prepared, clean the mill to avoid cross-contamination of differently flavored malts.
- Pour the entire sample into the filter at once so that the grains settle; filter paper should not have any aromas.
The result is wort that serves approximately 6-8 people and is ideal for evaluating aroma, flavor, mouthfeel, and color of specific malts.
See also:
- This Breiss blog post by Cassie Poirier and this BeerSmith podcast (~19:20 minutes in): https://youtu.be/KbHVk5oafw4?t=19m30s.
- Breiss video demonstrating the Hot Steep Method:
MBAA Podcasts
- Simplifying the understanding of malt certificates of analysis by Joe Hertrich Part 1, Part 2, Part 3, and Part 4.
- Malt flavor development by Joe Hertrich Part 1, Part 2, and Part 3.
- Kilned vs Roasted Malts (also a discussion of the ASBC Hot Steep Method).
- The history of 6-row and 2-row barley in the US with Joe Hertrich: Part 1, Part 2, and Part 3.
Blogs
- Brewing Beer the Hard Way (home malting) see also his Youtube channel.
- "Dextrins and Mouthfeel", by Scott Janish; a review of the science of dextrins and mouthfeel in beer, and an experiment.
- Briess blog article on a thought provoking perspective on using cold steeping methods in brewing by Dan Bies.
Malting and Growing Barley
See Also
MTF Posts
Additional Articles on MTF Wiki
- Culturing Lacto From Grains
- Sour Mashing
- Butyric Acid
- Isovaleric Acid
- Cereal Mashing
- Lactobacillus
- Pediococcus
External Resources
- Homebrew Talk Wiki page on Grain.
- Barley World, Oregon State University barley research website.
- Phenol-Explorer site that discusses phenols of some adjunct grains.
- "Brewing With Wheat", BYO article from September 1996 by Jeff Frane.
- "Brewing Beer the Hard Way" - blog about growing barley and home malting and youtube home malting videos.
- All Breiss Technical Presentations.
References
- ↑ Enhancing the Microbiological Stability of Malt and Beer — A Review. Anne Vaughan, Tadhg O'Sullivan, Douwe Van Sinderen. 2005. DOI: https://doi.org/10.1002/j.2050-0416.2005.tb00221.x.
- ↑ Manuel Taboada. Milk The Funk Facebook post on quinoa. 09/14/2017.
- ↑ Microbial Gluten Reduction in Beer Using Lactic Acid Bacteria and Standard Process Methods. Brett F. Taubman, Stephan Sommer, Jacob Edwards, Travis Laws, Logan Hamm, and Brenton A. Frank. 2018. DOI: https://doi.org/10.1094/TQ-55-1-0305-01 .
- ↑ "094: Microbial Gluten Reduction in Beer Using Lactic Acid Bacteria and Standard Process Methods". MBAA podcast. 06/25/2018.
- ↑ Joe Morris. Milk The Funk Facebook thread about gluten free resources. 09/11/2018.
- ↑ Ionic composition of beer worts produced with selected unmalted grains. Monika Sterczyńska, Marta Stachnik, Aleksander Poreda, Katarzyna Pużyńska, Joanna Piepiórka-Stepuk, Grzegorz Fiutak, Marek Jakubowski. 2020. DOI: https://doi.org/10.1016/j.lwt.2020.110348.
- ↑ N-nitrosodimethylamine in Spanish beers. M. Izquierdo-Pulido, J.F. Barbour, R.A. Scanlan. 1994.
- ↑ Nitrosamines. Wikipedia. Retrieved 11/15/2020.
- ↑ Konstantin Bellut. Milk The Funk Facebook group post about Bellut et al (2019). 10/11/2019.
- ↑ 10.0 10.1 10.2 10.3 "On the suitability of alternative cereals, pseudocereals and pulses in the production of alcohol-reduced beers by non-conventional yeasts." Konstantin Bellut, Maximilian Michel, Martin Zarnkow, Mathias Hutzler, Fritz Jacob, Kieran M. Lynch, Elke K. Arendt. Eur Food Res Technol (2019). DOI: https://doi.org/10.1007/s00217-019-03372-3.
- ↑ 11.0 11.1 11.2 11.3 Microflora during Malting of Barley: Overview and Impact on Malt Quality. A. Justé, S. Malfl iet, M. Lenaerts, L. de Cooman, G. Aerts, K. A. Willems and B. Lievens. 2011.
- ↑ Bacterial community dynamics during industrial malting, with an emphasis on lactic acid bacteria. A. Justé, S. Malfliet, M. Waud, S. Crauwels, L. De Cooman, G. Aerts, T.L. Marsh, S. Ruyters, K. Willems, P. Busschaerta, B. Lievens. 2014.
- ↑ The Good Scents Co. 2,3-butane diol. Retrieved 10/15/2015.
- ↑ The Good Scents Co. Acetoin. Retrieved 10/16/2015.
- ↑ Synthesis of Aroma Compounds By Wort Enterobacteria During First Stage of Lambic Fermentation. H. Martens, E. Dawoud, and H. Verachtert. Jan 1992.
- ↑ 16.0 16.1 16.2 The Microbiology of Malting and Brewing. Nicholas A. Bokulicha, and Charles W. Bamforth. June 2013.
- ↑ WORT ENTEROBACTERIA—A REVIEW. F. G. Priest, M. A. Cowbourne and J. S. Hough. 2013.
- ↑ 18.0 18.1 Bart Lievens - Bacterial community dynamics during industrial malting, at the Belgian Brewers Conference 2015.
- ↑ Lactobacillus plantarum and Pediococcus pentosaceus Starter Cultures as a Tool for Microflora Management in Malting and for Enhancement of Malt Processability. ARJA LAITILA, HANNELE SWEINS, ARVI VILPOLA, ERJA KOTAVIITA, JUHANI OLKKU, SILJA HOME, AND AULI HAIKARA. 2006.
- ↑ Mapping microbial ecosystems and spoilage-gene flow in breweries highlights patterns of contamination and resistance. Nicholas A Bokulich, Jordyn Bergsveinson, Barry Ziola, David A Mills. March, 2015.
- ↑ Biological Acidification of a Mash Containing 20% Barley Using Lactobacillus amylovorus FST 1.1: Its Effects on Wort and Beer Quality. Deirdre P. Lowe and Helge M. Ulmer. 2005.
- ↑ THE ROLE OF BACILLUS spp. IN N-NITROSAMINE FORMATION DURING WORT PRODUCTION N. A. Smith and P. Smith. 1992.
- ↑ Butyric Acid Off-Flavors in Beer: Origins and Control. D. B. Hawthorne, R. D. Shaw, D. F. Davine, and T. E. Kavanagh, Carlton. 1991.
- ↑ Genomics of Weissella cibaria with an examination of its metabolic traits. Kieran M. Lynch, Alan Lucid, Elke K. Arendt, Roy D. Sleator, Brigid Lucey and Aidan Coffey. 2015. DOI: 10.1099/mic.0.000053.
- ↑ "Salmonella_bongori". Wikipedia. Retrieved 06/15/2017.
- ↑ 26.0 26.1 26.2 26.3 26.4 "Mapping the sour beer microbiome". Matthew Bochman and Jeff Young. Experiment.com. 2017. Retrieved 06/15/2017.
- ↑ Edwards, John. Milk The Funk facebook group. 04/24/2017.
- ↑ Private correspondence with Jeff Young by Dan Pixley. 06/29/2017.
- ↑ Poirier, Cassie. "Malt Sensory Methods". Briess website. 02/08/2016. Retrieved 07/26/2018.