Difference between revisions of "Nonconventional Yeasts and Bacteria"
| Line 37: | Line 37: | ||
| Lallemand Brewing || Sourvisiae® || ''Saccharomyces cerevisiae'' || || || || A bioengenered strain of ''S. cerevisiae'' that produces lactic acid and no off-flavors within 5 days of fermentation <ref>[https://www.lallemandbrewing.com/en/united-states/news/launch-of-lactic-acid-producing-yeast-sourvisiae/ "Launch of lactic-acid producing yeast – Sourvisiae®" lallemand Brewing blog. 09/12/2019. Retrieved 09/12/2019.]</ref>. See also this [https://www.facebook.com/groups/MilkTheFunk/permalink/2907116972649831/ MTF thread]. | | Lallemand Brewing || Sourvisiae® || ''Saccharomyces cerevisiae'' || || || || A bioengenered strain of ''S. cerevisiae'' that produces lactic acid and no off-flavors within 5 days of fermentation <ref>[https://www.lallemandbrewing.com/en/united-states/news/launch-of-lactic-acid-producing-yeast-sourvisiae/ "Launch of lactic-acid producing yeast – Sourvisiae®" lallemand Brewing blog. 09/12/2019. Retrieved 09/12/2019.]</ref>. See also this [https://www.facebook.com/groups/MilkTheFunk/permalink/2907116972649831/ MTF thread]. | ||
|- | |- | ||
| − | | Lallemand Wine || Biodiva™ || ''Torulaspora delbrueckii'' || || || || Marketed as a wine yeast to be used in a sequential pitch with regular wine yeast. Pitch this first until 2° Brix have been fermented, then add wine yeast. See the [https://catalogapp.lallemandwine.com/uploads/yeasts/docs/d441f52d43e8c74d0071debbd7ff415c479e1fdf.pdf Lallemand data sheet] for more information. | + | | Lallemand Wine || Biodiva™ || ''Torulaspora delbrueckii'' || || || || Marketed as a wine yeast to be used in a sequential pitch with regular wine yeast. Said to increase mouthfeel in wine. |
| + | Pitch this first until 2° Brix have been fermented, then add wine yeast. See the [https://catalogapp.lallemandwine.com/uploads/yeasts/docs/d441f52d43e8c74d0071debbd7ff415c479e1fdf.pdf Lallemand data sheet] for more information. | ||
|- | |- | ||
| [[Mainiacal Yeast]] || Phenolic Funk Warrior || Pichia m, Pichia a, POF+ Saccharomyces cerevisiae x2. || 75-85% || | | [[Mainiacal Yeast]] || Phenolic Funk Warrior || Pichia m, Pichia a, POF+ Saccharomyces cerevisiae x2. || 75-85% || | ||
Revision as of 12:33, 28 January 2020
Nonconventional Yeasts and Bacteria are yeasts and bacteria genera that haven't been greatly explored in alcoholic fermentation but might prove to be worth exploration. This page contains anecdotal information, as well as scientific information that might prove useful for brewers who are looking to brew with microbes that don't include the typical lab yeasts and bacteria for sour/mixed fermentations. For yeasts and bacteria that are more often used in sour and mixed fermentations, see Saccharomyces, Brettanomyces, Lactobacillus, Pediococcus, Kveik, and Mixed Cultures.
For a family tree of yeast, see this diagram.
- To do: - https://www.sciencedirect.com/science/article/pii/S0740002018309778 - https://www.tandfonline.com/doi/full/10.1080/03610470.2019.1569452 - https://www.facebook.com/groups/MilkTheFunk/permalink/2576419979052867/ - https://onlinelibrary.wiley.com/doi/full/10.1002/jib.381 - https://www.sciencedirect.com/science/article/pii/B978012816678900014X - https://www.researchgate.net/publication/337907047_Screening_for_the_Brewing_Ability_of_Different_Non-Saccharomyces_Yeasts - https://onlinelibrary.wiley.com/doi/abs/10.1111/ijfs.14399
Under progress
Contents
- 1 Commercial Cultures
- 2 General Information
- 3 Yeasts
- 4 Bacteria
- 5 About Health Concerns
- 6 Potential references
- 7 See Also
- 8 References
Commercial Cultures
For mixed cultures that contain only the genera Brettanomyces, Lactobacillus, and/or Pediococcus, see Mixed Cultures Chart.
| Lab Name | Product Name | Taxonomy | Attenuation | Flocculation | Starter Note | Fermentation/Other Notes |
|---|---|---|---|---|---|---|
| AEB | Levulia Alcomeno | Lachancea thermotolerans (formerly classified as Kluyveromyces thermotolerans [1])) | Low (for maltose) | Alcohol tolerance: 7.2%. Medium nitrogen demands, very low production of acetic acid, but reportedly produces lactic acid. Initially developed by AEB for natural wine fermentation.
See also:
| ||
| East Coast Yeast | ECY31 Senne Valley Blend | Debaryomyces, Priceomyces, Wickerhamomyces, Pichia, lactic acid bacteria, a wild Saccharomyces, and B. bruxellensis | Intended for primary fermentations due to the portfolio of wild oxidative yeasts. Emulating ales by employing similar microflora recently observed from a prominent Lambic producer, this new blend includes various unique yeasts (Debaryomyces, Priceomyces , Wickerhamomyces, and Pichia). Contributes to aroma through the production of volatile compounds and higher terpenol flavors. Rounding out the blend : Lactic bacteria, Wild-type Saccharomyces from the Senne Valley, and multiple strains of Brettanomyces bruxellensis. Recommended to be aged a minimum of 1 yr. Extremely wild with notes of smokey barnyard, musty funk, and ethyl acetate [2]. | |||
| Fermmento Labs | FB6 Lambic & Sours | Pediococcus pentosaceus, Lactobacillus pentosus, Lactobacillus plantarum, Brettanomyces bruxellensis, Candida colliculosa, Kluyveromyces thermotolerans, Kloeckera africana, Hanseniaspora uvarum, Hanseniaspora apis, Belgian ale yeast, and sherry Flor yeast. | Ferments at 20-28°C [3]. | |||
| Fermmento Labs | FB10 Fruteira | Brettanomyces anomalus, Torulaspora delbruekii, Lanchancea thermotolerans, Kloeckera africana, and Hanseniaspora uvarum | Ferments at 28-30°C [3]. | |||
| Lallemand Brewing | Sourvisiae® | Saccharomyces cerevisiae | A bioengenered strain of S. cerevisiae that produces lactic acid and no off-flavors within 5 days of fermentation [4]. See also this MTF thread. | |||
| Lallemand Wine | Biodiva™ | Torulaspora delbrueckii | Marketed as a wine yeast to be used in a sequential pitch with regular wine yeast. Said to increase mouthfeel in wine.
Pitch this first until 2° Brix have been fermented, then add wine yeast. See the Lallemand data sheet for more information. | |||
| Mainiacal Yeast | Phenolic Funk Warrior | Pichia m, Pichia a, POF+ Saccharomyces cerevisiae x2. | 75-85% | This blend is best used with a Brett secondary. High creation of phenols along with higher hopping rates(aged hops are best) will give Brett more to feed on and allow it to be more expressive. Choosing the Brett strain will also vastly change what the outcome is. | ||
| Mainiacal Yeast | MYLAY2 | Wickerhamomyces anomolus | 80-100% | A very unique strain. This is part of our Lactic Acid Yeast strains. These LAY type strains were pioneered by Wild Pitch Yeast. They are very unique in the fact that as they ferment they also produce lactic acid. So essentially it’s a yeast that can make a sour beer all on its own! We mutated this strain to our needs over a year. It's now more acid tolerant(there was a real problem with viability after a fermentation), more tolerant of temperates, better suited in anaerobic environments, and less stressed by a maltose filled environment. That being said, its still not as fond as a wort environment but its more tolerant then it was originally. There are many strains of Brettanomyces in the brewing world that are misidentified and are actually Wickerhamomyces a. This particular strain is great for adding what we know as a "Brett like quality" while also souring the beer. | ||
| Mainiacal Yeast | Lachancea thermotolerans | MYLAY5 | 70-95% | The famed Lachancea t species! This was the first species announced to be proof of yeast strains that could produce a significant amount of lactic acid. Like most of our Lachancea strains, this strain also produces higher amounts of glycerol making for a better body beer as well as amazing head formation and retention. It lends hints of stone fruit but is very neutral and balanced in flavor. | ||
| Mainiacal Yeast | Lachancea thermotolerans | MYLAY8 | 75-95% | Another one of our Lachancea t species. This was the first species announced to be proof of yeast strains that could produce a significant amount of lactic acid. Like our other Lachancea strains this strain also produces higher amounts of glycerol making for a better body beer as well as amazing head formation and retention. This one tends to sour a bit less then our other Lachancea t strain and lends a clean ale profile with hints of fruit esters. | ||
| Mainiacal Yeast | Earth Bender | Tolurspora delbrueckii | 62-80% | This particular strain of T. delbrueckii brings a earthy character but has a ethanol tolerance of around 5% so is best used when co pitched with another strain or low gravity beers. It tends to be more earthy when used co pitched. | ||
| Mainiacal Yeast | MYTD1 | Tolurspora delbrueckii | 65-78% | This strain is very hefeweizen like. It lends notes of cloves and light bananas while also having a slight rustic farmhouse feel. It's ethanol tolerance is around 6% so should not be used in high gravity beers unless co pitched with a strain that can attenuate out. | ||
| Mainiacal Yeast | Basement Dweller | Debaryomyces hansenii | 10-20% | This strain needs to be used in conjuction with another primary ferment. It will not fully attenuate but adds a funky quality to whatever its used in. It can be used pre ferment, co ferment, or post fermentation. Like most other Debaryomyces species this strain has a high salinity tolerance. | ||
| Mainiacal Yeast | MYOO1 | Oenococcus oeni | Less then 5% | A lactic acid bacteria that can also convert malic acid to lactic acid. This species is generally used in wine making to soften the malic acid character. However some strains can also metabolize maltose making it a viable souring bacteria. This strain can do so and adds a light stone fruit note to the beer. Keep in mind if fruiting a beer while this is present it will consume malic acid from the fruit creating more lactic acid. | ||
| Mainiacal Yeast | MYOO2 | Oenococcus oeni | Less then 5% | A lactic acid bacteria that can also convert malic acid to lactic acid. This species is generally used in wine making to soften the malic acid character. This strain cannot metabolize maltose so should be used after a beer has had some sort of fruit added or other carbon source of glucose/malic acid. We find this also pairs well with other bacteria strains. | ||
| Mainiacal Yeast | Alternative to Alternatives | Currently this blend contains Zygosaccharomyces , Lachancea f, Lachancea t, Wickerhamomyces a, S. japonicus, Hanseniaspora v, Pichia a, Pichia m, 2 strains of Toluraspora d, Debaryomyces h, 3 strains of Oenococcus o, and Weissella c. | 80-100% | Low | One of our most unique blends. This includes all of our yeast and bacteria strains that are not Sacc, Brett, Lacto, or Pedio. This blend will continue to change as we added new microbes to our bank. This blend is very unique as it changes severely depending on its environment and carbon sources. Can serve as a primary pitch with no additional brewer's yeast, or pitch additional brewer's yeast to suppress some yeast expression in this blend which results in more nuanced flavors [5]. | |
| Mainiacal Yeast | MYLAY4 | Lachancea fermentati | 75-95% | Medium | A LAY strain that just like the rest has been mutated to our needs. What is interesting about this strain is it is quite neutral in flavor and doesn't produce as much lactic acid as most of our LAY strains. It leaves the beer slightly tart usually around 3.7-3.9 pH. This strain also produces more glycerol then most yeast strains leaving more body as well as an amazing head creation and retention. | |
| Mainiacal Yeast | Hardened Spaniard | Zygosaccharomyces parabailii | 76-100% | This species is generally found in Sherry wines. It's most commonly referred to as a "Flor" yeast. Is is a oxidative yeast that also forms a heavy pellicle with access to O2. It's best used in secondary environments but can be used in primary. It lends notes of fresh cut apples and earthy/hazelnut like flavors. | ||
| The Yeast Bay | Metschnikowia reukaufii | Metschnikowia reukaufii | 20-25% (cannot utilize maltose) | Med-High | M. reukaufii is a nectar specialist that was isolated from flowers in the Berkeley Hills of California. Evolutionarily, these yeast likely evolved to produce a more odorous and attractive nectar for pollinators by enzymatically altering otherwise inodorous nectar compounds like glycosides.
While only attenuating to 20-25% in brewer’s wort and not utilizing maltose or maltose polymers, in co-fermentations it has been shown to drop gravity and pH of the fermentation faster, accentuate and modulate the flavor and aroma profile and soften the perceived bitterness of the finished product. This accentuation of the aroma profile is likely due to not only the complex though rather subdued fruit cocktail ester profile of M. reukaufii, but also to the production of glucosidases (exhibited by others in the genus) that utilize hop glycosides as substrate to free flavor active molecules from the sugars to which they are bound. This strain MUST be used in conjunction with other yeast that can ferment brewer’s wort that is capable of fermenting maltose. Count: ~10 billion cells/vial [6]. See also Metschnikowia reukaufii. | |
| White Labs | Various | Various | See https://www.whitelabs.com/yeast-vault | |||
| Wild Pitch Yeast | Yeast YH2 | Hanseniaspora uvarum | 45% | This wild cousin of Saccharomyces cerevisiae that is often found in wine fermentations was isolated from a serviceberry in Bloomington, IN in 2014. This yeast produces a yeasty aroma and lends a pleasant tart, spicy flavor to beer. It is recommended for a mixed fermentation with a neutral, more attenuative S. cerevisiae strain [7]. Produces lactic acid (down to pH 3.2 in some cases) and ethanol (up to 8-9% ABV) at the same time and is hop tolerant up to at least 75 IBU [8]. | ||
| Wild Pitch Yeast | Yeast YH39 | Lanchancea thermotolerans | 40% | This wild cousin of Saccharomyces cerevisiae was isolated from a chestnut oak tree in Bloomington, IN in 2014. This yeast produces a mild yeasty aroma and beers that flavors that are Belgian- and saison-like with a subtle spice. It is recommended for a mixed fermentation with a neutral, more attenuative S. cerevisiae strain [7]. | ||
| Wild Pitch Yeast | Yeast YH52 | Torulaspora delbrueckii | 63% | This wild cousin of Saccharomyces cerevisiae was isolated from a white oak tree on the Indiana University campus in Bloomington, IN in 2014. This yeast produces a Belgian phenolic character and an original bubblegum flavor [7]. |
General Information
Killer Toxins
Many genera of yeast and bacteria produce toxins that other strains or species are sensitive to. See Killer Yeast strains and Bacteriocins for more information.
Yeasts
Cyberlindnera spp.
The organoleptic properties of several species from the genus Cyberlindnera have been studied with promising results for non-alcoholic wort fermentation. These results can also be applied to alcoholic mixed fermentation. Bellut et al (2019) reported pleasant fruity characteristics from 4 out of 6 strains of various species from this genus (the other two strains produced unpleasant solvent-like aromas). They did not produce significant alcohol (18-25% attenuation) and could not ferment maltose or maltotriose. While they all could ferment glucose, four of the six strains fermented sucrose and only one strain fermented fructose. IBU up to 100 were tested against these strains' ability to grow, and that high of an IBU had no reported effects. All strains tested did not produce phenols (POF-). Four of the six strains could tolerate up to 5% ABV, and two of those tolerated up to 7.5% ABV (the higher ABV resulted in longer lag times by 24-48 hours). All of the six strains tolerated a pH of 4 and five of the six strains tolerated a pH of 3 (only at a pH of 3 did the strains see an extended lag time during growth, from 12-78 hours depending on the strain), although the presence of lactic acid instead of the hydrochloric acid used in this study could have a stronger inhibitory effect. Glycerol production was low (0.18 - 0.36 mg/L). Two of the strains produced very high levels of acetaldehyde (9.7 and 8.1 mg/L), and one strain produced very high ethyl acetate (65.7 mg/L compared to 4.9-22.6 mg/L). Isoamyl acetate was produced at a wide range between the different strains, from 1.67 mg/L to 0.15 mg/L [9].
Overall, the species that were favorable with fruity-like characteristics were: C. jadinii, C. mrakii, and two strains of C. subsufficiens. The strains that were characterized as unpleasant were C. misumaiensis (described as solvent-like due to high levels of ethyl acetate) and C. fabianii (described as cabbage-like due to an unidentified aroma compound) [9].
Cyberlindnera subsufficiens
Due to the positive aroma characteristics and fermentation abilities in wort of one of the two strains of C. subsufficiens tested by Bellut et al. (2019) as mentioned above, the researchers chose to test for optimal fermentation conditions for this strain. It was determined that the highest fruitiness was achieved at the lowest fermentation temperature tested (62°F/17°C) and the lowest pitching rate (1 x 1010 cells/mL), while the least fruitiness was achieved at the warmest fermentation temperature (80.6°F/27°C) and the highest pitching rate (6 x 1010 cells/mL), following a linear model. They brewed a non-alcoholic beer with this strain and compared the sensory to two other non-alcoholic beers (NAB) and found that the NAB brewed with C. subsufficiens tasted less like unfermented wort and more fruity and tropical (more specifically described as "banana, pear, mango, maracuja, lychee") than the other two commercial NAB products. The fermentation time took 6 days at the warmest temperature (80.6°F/27°C) and 13 days at the coolest temperature (62°F/17°C). For a mixed fermentation with S. cerevisiae, Bellut suggests fermenting C. subsufficiens at 68°F/20°C for 2-3 days, and then pitching S. cerevisiae and/or other microbes [9][10].
Debaryomyces spp.
Debaryomyces is a genus of yeast commonly referred to as a spoilage yeast [11]. The non-pathogenic species D. hansenii is commonly found in cheese and is an osmotolerant, halotolerant, and xerotolerant (tolerant high amounts of salt and sugar, and low amounts of water) [12]. Debaryomyces are associated with natural fermentation, and tend to develop during the maturation of beer [13]. Many species of Debaryomyces have been to biotransform monoterpenes found in hop oils (see Hop Biotransformations).
Recently it was found living cells of a Debaryomyces species in a bottle of porter found in a shipwreck under the English Channel that was dated to 1825. It is currently unknown how this yeast might have affected the flavor of the historical porter, but the characterization of this yeast is underway by Brewlab in the UK [13].
Some species of Debaryomyces can produce exopolysaccharides (EPS), which are extracellular (produced and expelled outside of the cell) polymers of monosaccharides connected by glycosidic bonds with a degree of polymerization higher than 10. EPS assists in producing biofilms for microorganisms. It is possible that EPS from yeast could make beer "ropy" or "sick", similar to Pediococcus [14].
Debaryomyces hansenii
D hansenii is the most prevalent yeast in dairy and meat products as well as early stages of soy sauce fermentation. Various isolates exist originating from cheese, sake moto, edomiso, rennet, psoriasis, infected hands and salmon. In general, D. hansenii can be found in habitats with low water activity as well as in products with high sugar concentrations. Although D. hansenii is considered a non-pathogenic yeast, various clinical cases of D. hansenii exist. This yeast was originally isolated from saline environments and is maybe one of the most osmotolerant (can tolerate high levels of salt and sugar) yeasts in existence. [15]
See also:
General Information
As already mentioned, D. hansenii can tolerate very high levels of salt. Some sources cite salinity levels up to 24% whereas Saccharomyces cerevisiae commonly tolerate levels up to 10%. Such high tolerances are not that common in living organisms and can be used on industrial scale by cultivating D. hansenii at high salt levels to prevent the growth of other yeasts (quasi non-sterile production conditions). Beside dealing with high osmolarities, D. hansenii secrete toxins capable of killing other yeasts. [15]
Although this yeast is already an extremophile(an organism that thrives in physically or geochemically extreme conditions that are detrimental to most life on Earth) in terms of osmolarity, it does not stop there. Besides the normal sugars, D. hansenii is capable of metabolizing n-alkanes, melibiose, raffinose, soluble starch, inositol, xylose, lactic acid and citric acid. Furthermore, this yeast can form arabitol(a sugar alcohol) as well as riboflavin (vitamin B2). D. hansenii is therefore used on industrial scale to produce vitamin B2 and has a big potential for other biotechnological processes. [15]
D. hansenii is a very common yeast in cheeses and seems to have a major impact on the development of the microflora as well as the taste. As previously mentioned, D. hansenii can metabolize lactic acid, citric acid and galactose. The metabolization of lactic acid by yeasts has been shown to have an impact on the bacterial flora of the cheese in types such as Limburger, Tilsiter, Port Salut, Trappist, Brick and the Danish Danbo. Furthermore, D. hansenii forms volatile compounds associated with a “cheesy” flavor. For example, D. hansenii seems to have a major role in the development of Cheddar and Camembert cheese by synthesizing S-methylthioacetate (most prevalent volatile sulfur compound found in cheese).[15]
Debaryomyces nepalensis
Debaryomyces nepalensis is an osmotolerant yeast isolated from rotten apples that is known to utilize both hexoses and pentoses and produce industrially important metabolites like ethanol, xylitol and arabitol. [16]
Sugar Utilization and Ethanol Creation
| Carbon Source | Carbon Source Consumed (g/L) | Ethanol Created (g/L) |
|---|---|---|
| Sucrose | 82.00 | 9.90 |
| Glucose | 84.75 | 9.05 |
| Arabinose | 86.70 | 2.43 |
| Fructose | 80.40 | 9.84 |
| Glycerol | 50.60 | 0.77 |
General Information
Effect of nitrogen sources
The organism was grown in the presence of different sources of nitrogen like, ammonium sulphate, nitrates and nitrites along with yeast extract and its ability to produce ethanol and arabitol was studied. Among them ammonium sulphate served as the best nitrogen source, whereas, in the presence of nitrites and nitrates, the organism failed to metabolize glucose efficiently. Yeast extract proved to be an integral source of amino acids and other vitamins for growth, without which, the organism had low efficiency for its metabolism. [16]
Hanseniaspora
Wines fermented with a combination of M. pulcherrima, H. uvarum, S. cerevisiae, and lactic acid bacteria, had a slightly lower ethanol percent, but a higher phenolic acid content and slightly better mouthfeel [17]. Hanseniaspora osmophilia and H. valbyensis have been found to dominate indigenously fermented cider gum sap in Australia, and have been found to tolerate up to 11% ABV and cold temperatures.
Some species of this genus cannot ferment maltose or maltotriose, which make up the majority of sugar in brewer's wort. For example, Bellut et al. (2018) found that one strain each of the species H. vineae and H. valbyensis that were both isolated from kombucha could not ferment these complex sugars (including sucrose), but could ferment cellobiose. This is due to the lack of a maltose transporter and the enzyme maltase. As such, they have been proposed as being potentially useful in non-alcoholic beer fermentation. Additionally, these species were able to grow in 7◦ Plato wort with a range of IBU (50 IBU was the maximum IBU tested), indicating that IBU's don't impact the growth of these species. They also lacked the ability to produce phenols. H. vineae was moderately flocculant, while H. valbyensis had poor flocculation, with the flocculation depending on the FLO gene and the presence of calcium in the wort (the same as Saccharomyces). They produced much less higher alcohols (n-propanol, isobutanol, and isoamyl alcohol) than the WLP001 control yeast. H. vineae produced slightly more ethyl acetate than WLP001 (6 mg/L vs 4.05 mg/L), while H. valbyensis produced above threshold diacetyl (0.21 mg/L), however, due to the worty taste of the Hanseniaspora species which didn't highly attenuate the wort, the sensory differences were negligible. Both species of Hanseniaspora produced lower levels of ethyl formate and about half the amount of acetaldehyde as WLP001 [18].
See also:
- https://www.mdpi.com/2311-5637/4/3/76
Kluyveromyces
Many species of Kluyveromyces have been to biotransform monoterpenes found in hop oils (see Hop Biotransformations).
Lachancea
Lachancea thermotolerans
Formerly classified as Kluyveromyces thermotolerans [19], L. thermotolerans is a species of yeast that has been found to produce small amounts of lactic acid during fermentation (also known as "lactic acid yeast").
The optimal fermentation range is reported to be between 61-68°F (16-20°C) for one strain that Bryan of Sui Generis blog has worked with, 70-72°F (21-22°C) for another strain that DeWayne Schaaf/Justin Amaral have traded on MTF, and 74°F (23°C) for a third strain that Justin Amaral has worked with. Many strains may die at temperatures as low as 84°F (28°C). Some other species of Lachancea die at 20°C (68°F), hence the species name "thermotolerans" [20].
The amount of time needed to ferment wort with L. thermotolerans appears to be strain dependent. The strain from DeWayne Schaaf, for example, takes around 3 weeks to finish fermenting, and ends up at around 3.7-3.9 pH (not a lot of lactic acid is produced). Bryan of Sui Generis blog reported that his strain of L. thermotolerans takes about 2 weeks to ferment, but the resulting beer improves flavor-wise with a few weeks to months of aging [21].
Many other strains have a lower fermentation capacity. Because of this, studies in wine have focused on co-fermentation with Saccharomyces in order to reduce the pH of the wine and provide fruity ethyl lacate esters. Three strains were tested by Dimizio et al. (2016) for their fermentation characteristics after 21 days of fermentation under different conditions. They found that all three strains fermented maltose at similar levels of S. cerevisiae, but none fermented maltotriose and other studies have tested strains that do not ferment maltose. The L. thermotolerans strains produced 6-12% less total ethanol than S. cerevisiae, showing that in general that this species has lower attenuation than brewers yeast. All three strains of L. thermotolerans produced lactic acid, but it took 21 days to achieve maximum lactic acid levels, and only one strain resulted in beers that were at a pH of 3.77 (the other strains produced beers that were at a pH of 4.11 and 4.28, which were similar to the S. cerevisiae strain that was tested). It was noted that other strains have been reported to produce a pH of 3.6, so the ability of L. thermotolerans to sour beer is widely dependent on strain. L. thermotolerans also produced significantly more glycerol than the beer yeast (between 65-75% more at day 21), which demonstrates that this species could be used to improve mouthfeel. Pitching rate didn't greatly affect the amount of lactic acid produced, although the lowest pitching rate tested produced slightly more lactic acid. Repitching up to five generations did not seem to have a great effect on viability and slightly improved its fermentation capability, and they were not noticeably affected by high IBU's (60) or low vs high oxygenation levels. L. thermotolerans did not have a negative effect on head retention, and behaved similarly to S. cerevisiae as far as flocculation. Overall, the levels of VDK's and diacetyl were lower than that of the tested strain of S. cerevisiae, however, another study showed that they were higher in wort that was highly saturated with oxygen. The sensory effects of L. thermotolerans were described as "positive", but the data was not shown in the study. At lower fermentation temperatures (16°C), the tasters described the beer as tasting "fruity, floral, sour, clove, melon, and strawberry". However, in another study of another strain that did not ferment well was described as "yielded strong, unpleasant phenolic aromas, notably 4-ethylphenol." This seems to indicate that L. thermotolerans generally produces phenols, although phenols were not measured in this study. One strain tested was shown to have higher beta-glucosidase activity, which could indicate that it could aid in the break down Glycosides in hops or fruit [19].
A study by Hranilovic et al. (2018) looked at the fermentation profile of 94 strains of L. thermotolerans from all over the world in Chardonnay grape juice. They found a wider range of fermentation profiles for different strains of this species. They achieved a range of 7.3 to 10.6% ABV, preferring glucose over fructose. Some strains produced extreme levels of glycerol (8.0 g/L) while others produced moderate amounts. 48 of the strains produced more lactic acid than they did glycerol, which is a significant amount of lactic acid, the highest produced being g/L. Some strains didn't produce nearly as much lactic acid though, producing as little as 1.8 g/L. Acetic acid production was insignificant in all strains tested, but they all produced low levels. They found that a wide range of secondary metabolites were produced, including high alcohols (hexanol, phenylethanol, methylbenzenemethanol, isobutanol, isoamyl alcohol, methyl-butanol, methyl-pentanol, ethylhexanol, butanol, nonanol, octanol, and decanol). Some strains produced high levels of hexanol and octanol. Many esters were produced as well, including ethyl propanoate, ethyl octanoate, ethyl decanoate, ethyl 9-decenoate, diethyl succinate, ethyl acetate, isobutyl acetate, isoamyl acetate, 2-phenylethyl acetate, and amyl lactate. Other aromatic compounds were also produced, including aldehydes, ketones, and the terpene citronellol. They found that some groups of strains produced some compounds more than others, indicating a potentially high degree of variability on the flavors produced by different strains of L. thermotolerans [22].
See also:
- Post 1 and Post 2 on Lachancea thermotolerans that can produce significant lactic acid without modification.
- MTF thread on an application from John Sheppard, Robert Dunn, Anne Madden from North Carolina State University to patent pitching this yeast into wort, which prevents other yeast labs from offering any other strains of this species and also prevents brewers from using this species from other sources. A follow up post on 09/01/2017 discusses Sheppard and Madden opening a business that claims patent on this yeast, and restricts competing breweries/yeast companies from using it. University of Sciences Philidelphia, via Matthew J. Farber's work, is also trying to claim patent on this process.
- Matt MIller's notes on a few species of Lactic Acid Yeast.
- Levulia Alcomeno (AEB) Yeast Trial On Cabernet Franc (2016); formerly classified and listed as Kluyveromyces thermotolerans.
- MTF thread by Jeremy Myers on making a saison style beer with Levulia Alcomeno.
(To do)
- Bochman et al. (2018) paper: https://www.sciencedirect.com/science/article/pii/S0740002017302952
Lachancea fermentati
Metschnikowia
Wines fermented with a combination of M. pulcherrima, H. uvarum, S. cerevisiae, and lactic acid bacteria, had a slightly lower ethanol percent, but a higher phenolic acid content and slightly better mouthfeel [17].
https://www.facebook.com/groups/MilkTheFunk/permalink/1862425643785641/
Metschnikowia reukaufii
Mrakia
Mrakia gelida
This species has been suggested to be good for low alcohol (~1.5% ABV) beers due to favorable flavor contributions and a lack of off-flavors like diacetyl production [23].
Pichia
Pichia is a genus of yeasts in the family Saccharomycetaceae with spherical, elliptical, or oblong cells. Pichia is a teleomorph, and forms hat-shaped, hemispherical, or round ascospores during reproduction. The anamorphs of some Pichia species are Candida species. The asexual reproduction is by multilateral budding. Pichia can be prolific pellicle-forming yeasts. [24] Many species of Pichia have been to biotransform monoterpenes found in hop oils (see Hop Biotransformations).
Some species of Pichia can produce exopolysaccharides (EPS), which are extracellular (produced and expelled outside of the cell) polymers of monosaccharides connected by glycosidic bonds with a degree of polymerization higher than 10. EPS assists in producing biofilms for microorganisms. It is possible that EPS from yeast could make beer "ropy" or "sick", similar to Pediococcus [14][25].

Pichia kluyveri
Proposed to be useful in the production of non-alcoholic beer. See this MTF thread on suspecting this yeast to be the one used in a recent Mikkeller non-alcoholic beer called "Henry and His Science".
This MTF thread by Brendan Pleskow explores the possibility of producing significant ethanol with this species using BSG Amylo™ enzyme.
Chr Hansen Frootzen® is claimed to be a high thiol producer.
Pichia kudriavzevii
P. kudriavzevii is a very abundant yeast found in soil, fruits, and various fermented beverages. It is ovoid to elongate in shape. So far, P. kudriavzevii is mainly associated with food spoilage to cause surface biofilms in low pH products. It is also known for known for creating a very heavy pellicle. [26]
Sugar Utilization
P. kudriavzevii can mainly metabolize glucose making it a non-viable strain for primary fermentations. During trials it was unable to metabolize galactose, sucrose, maltose, lactose, raffinose, and trehalose. [26] Interestingly, some strains of P. kudriavzevii can metabolize pentose sugars such as xylose [27].
Pichia apotheca
Pichia apotheca is a new hybrid species of Pichia which was identified in 2017. [28] Pichia apotheca was identified as a hybrid of Pichia membranifaciens and another unidentified species of Pichia.
Characterization
During the study, a fermentation using solely Pichia apotheca was conducted. The test wort used was at 13.75 degrees Plato and after 5 weeks at 13.61 degrees Plato. The alcohol by weight was found to be 0.02% after fermentation. In addition to this, percentages based on the entire contents of the wort showed that over five weeks, glucose levels dropped from 1.62% to 1.22%. The hybrid may incrementally breakdown maltotriose and fructose, dropping from 1.46% to 1.05% and 0.57% to 0.22% respectively, but did not appear to be able to reduce the levels of maltose. These results indicate that the Pichia hybrid did not significantly metabolize much of the available carbohydrates into alcohol within this wort environment. [28]
See also:
- MTF thread on characterizing the strain of P. apotheca found and distributed by Maitreya Dunham.
- BasicBrewing radio podcast interview with Maitreya and two brewers who brewed a beer and a mead with this yeast.
Pichia membranifaciens
Pichia anomala
Schizosaccharomyces
Schizosaccharomyces is a genus of fission yeasts. The most well-studied species is S. pombe. At present four Schizosaccharomyces species have been described (S. pombe, S. japonicus, S. octosporus and S. cryophilus). Like the distantly related Saccharomyces cerevisiae, Schizosaccharomyces is a significant model organism in the study of eukaryotic cell biology. It is particularly useful in evolutionary studies because it is thought to have diverged from the Saccharomyces cerevisiae lineage between 300 million and 1 billion years ago, and thus provides an evolutionary distant comparison.
Schizosaccharomyces japonicus
Justin Amaral's experiencing using S. japonicus https://www.facebook.com/groups/MilkTheFunk/permalink/1457271340967742/
Schizosaccharomyces pombe
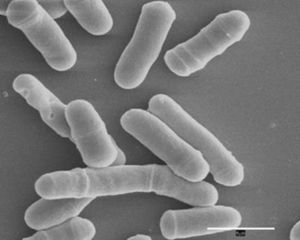
The fission yeast S. pombe is a unicellular eukaryote [29] that is rod shaped. They measure approximately 2 to 3 microns in diameter and 7 to 14 microns in length. S. pombe is usually found in sugar-containing fermentations of alcohol from subtropical regions. Even though its origin dates back to quite a long time ago, it was not widely known before the 1890’s. It was discovered in 1893 when a group working in a Brewery Association Laboratory in Germany was looking at sediment found in millet beer imported from East Africa that gave it an unsavory acidic taste. P. Lindner was the first to describe Schizosaccharomyces pombe. He chose as its epithet the Swahili word for beer, pombe. It was identified as yeast, and it became known as the fission yeast because it reproduces by means of fission unlike its relative Saccharomyces cerevisiae. The name Schizosaccharomyces was assigned to it because Schizo- means different, which had been previously used to describe other fission species. [30]
Dr. Matt Bochman has experimented fermenting beer with some strains of S pombe. He reported that a lot of sulfurous compounds were produced, but this could have been just his strains or his fermentation conditions [31].
Torulaspora delbrueckii
- to review: http://www.mdpi.com/2311-5637/4/2/22 - https://www.ncbi.nlm.nih.gov/pubmed/29492641 - https://www.facebook.com/groups/MilkTheFunk/permalink/2037872376240966/
Torulaspora delbrueckii is species of yeast, that is round to ovoid in shape and has been traditionally used in some wine fermentations to increase the complexity. Most of the commercial Torulaspora species and strains were isolated from soil, fermenting grapes (wine), berries, agave juice, tea-beer, apple juice, leaf of mangrove a tree, moss, lemonade and tree barks. Although it was said that most T. delbrueckii strains would not fully attenuate or tolerate higher alcohol contents it has been shown that this property is strain-dependent.
General Information
An analysis was done on 10 different T. delbruckii strains on various types of stress resistance as well as the ability to metabolize different carbon sources. The strains tested and the results are shown below. [32]
| Designation | Strain number/signature | Origin |
|---|---|---|
| T6 | RIBMa TdA | Wine |
| T9 | DSMb 70504 | Sorghum Brandy |
| T10 | CBSc 1146T | Unknown |
| T11 | TUMd 214 | Bottle (Pils beer, trace contamination, no beer spoilage observed) |
| T13 | TUMd TD1 | Wheat beer (starter culture) |
| T15 | TUMd 138 | Cheese brine |
| T17 | WYSC/Ge 1350 | Unknown |
| T18 | CBSc 4510 | Unknown |
| T19 | DSMb 70607 | Unknown |
| T20 | CBSc 817 | Unknown |
Hop Resistance
The resistance to alpha acids were also measured among these 10 strains using 0 PPM, 50 PPM, and 90 PPM. All strains were found to be resistant to these levels of alpha acids not affecting their growth. Some strains however were shown to have slower growth rates in the presence of 90 PPM and more [32]. One strain of Torulaspora delbrueckii was found to biotransform monoterpenes from hop oils (see Hop Biotransformations). Bellut et al. (2018) found that IBU's up to 50 had no impact on the growth of the strain of T. delbruekii that they tested, but they did not test the growth of this strain in wort above 50 IBU [18].
Ethanol Resistance
All 10 strains were also tested for their ability to grow in 5-10% ethanol content. The table below shows that all but one strain was able to grow in presence of 5% total alcohol but one thing they all shared in common is their inability to grow when in the presence of 10% alcohol. [32]
Growth (+) positive; (−) negative.
| Ethanol % | T6 | T9 | T10 | T11 | T13 | T15 | T17 | T18 | T19 | T20 |
|---|---|---|---|---|---|---|---|---|---|---|
| 5% | - | + | + | + | + | + | + | + | + | + |
| 10% | - | - | - | - | - | - | - | - | - | - |
Sugar Utilization
During fermentation trials of these 10 strains mentioned, sugar content was measured both before and after fermentation via HPLC. Tests showed the the sugar utilization of T. delbruekii is very strain dependent. All but one of the strains were shown to not ferment maltose and maltotriose. Although these tests do not show if these strains are able to utilize lactose, Eureka Brewing's blog mentions that they are unable to metabolize it.[33] The table below shows the percentages of sugars metabolized in the test wort by each strain. [32]
| Sugar Type | T6 | T9 | T10 | T11 | T13 | T15 | T17 | T18 | T19 | T20 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fructose (%) | 93.2 | 92.3 | 91.5 | 88 | 91.6 | 90.2 | 84.5 | 96.4 | 93.6 | 88.1 |
| Glucose (%) | 96.6 | 96.2 | 97 | 96.6 | 97.3 | 95.5 | 94.5 | 95.4 | 94.7 | 89.6 |
| Sucrose (%) | 82.4 | 86.4 | 79 | 95 | 84.6 | 75.2 | 78.3 | 72 | 73.7 | 84.7 |
| Maltose (%) | 3.3 | 94.8 | 5.8 | 6 | 1.8 | 0.3 | 0.9 | 2.5 | 0.7 | 0.3 |
| Maltotriose (%) | 3 | 58.9 | 1.6 | 4.2 | .5 | 1.3 | 2.4 | 0.1 | 0.1 | 3.6 |
Bellut et al. (2018) found that a strain of T. delbruekii isolated from kombucha could ferment glucose, fructose, sucrose, melibiose, and raffinose, but could not ferment maltose, maltotriose, or cellobiose [18].
Cross Resistance
Again, all 10 strains growth was tested but this time with the presence of both 5% ethanol as well as 50 and 90 PPM of iso-alpha acid concentrations. Below you can see that with a combination of these two factors, growth was hindered in quite a few strains. [32]
Growth (+) positive; (−) negative.
| IBU/ethanol % (v/v) | T6 | T9 | T10 | T11 | T13 | T15 | T17 | T18 | T19 | T20 |
|---|---|---|---|---|---|---|---|---|---|---|
| 50/5 | - | + | - | + | - | + | + | + | + | - |
| 90/5 | - | - | - | + | - | + | + | + | + | - |
Flavor Compounds
Bellut et al. (2018) found that a strain of T. delbruekii isolated from kombucha produced much less higher alcohols than WLP001 (n-propanol, isobutanol, and isoamyl alcohol) and also much lower esters, but slightly higher diacetyl and acetaldehyde. It also did not produce phenols and had moderate flocculation [18].
MTF Threads
Wickerhamomyces spp.
Some species can produce lactic acid. One strain of Wickerhamomyces anomalus isolated from a distillery was found to produce significant levels of ethanol and is ethanol tolerant. Another isolate attenuated wort 83% of wort, and was reported to ferment maltotriose, which is very rare for yeast species other than S. cerevisiae [34]
See also:
Zygosaccharomyces spp.
Zygosaccharomyces spp. belongs to the group of hemiascomycetous (class of fungi in which no ascocarps are formed) yeasts with a high tolerance to osmotic stress. This typical feature enables it to grow in environments with high concentrations of salts and/or sugars, i.e. under conditions restrictive to most other yeast species. Z. bailii, Z. bisporous, Z. rouxii, and Z. florentinus are species which have been isolated in grape musts or wine. Some strains can be very tolerant to a wide range of stressors, including 50% sugar, 2.5% acetic acid, 18% ethanol, and pH 2.0. It is also resistant to preservatives commonly used in beverage production such as SO2. They are commonly mentioned as part of the "Flor" present in Sherry wines.
Zygosaccharomyces parabailii
Zygosaccharomyces bailii
Some species of this genus cannot ferment maltose or maltotriose, which make up the majority of sugar in brewer's wort. For example, Bellut et al. (2018) found that one strain that was isolated from kombucha could not ferment these complex sugars. This is due to the lack of a maltose transporter and the enzyme maltase. It also could not ferment melibiose, raffinose, or cellobiose, but could ferment glucose, fructose, and sucrose. As such, they have been proposed as being potentially useful in non-alcoholic beer fermentation. Additionally, these species were able to grow in 7◦ Plato wort with a range of IBU (50 IBU was the maximum IBU tested), indicating that IBU's don't impact the growth of these species. They also lacked the ability to produce phenols. It was described as moderately flocculant, with the flocculation depending on the FLO gene and the presence of calcium in the wort (the same as Saccharomyces). They produced much less higher alcohols (n-propanol, isobutanol, and isoamyl alcohol) than the WLP001 control yeast and fewer esters, acetaldehyde, and diacetyl than WLP001 [18].
Zygosaccharomyces kombuchaensis
Bellut et al. (2018) found that a strain of S. kombuchaensis isolated from kombucha performed much the same as the above described strain of Z. bailii, except it was able to ferment raffinose [18].
Zygosaccharomyces rouxii
Bacteria
Leuconostoc
Leuconostoc is a genus of Gram-positive bacteria, placed within the family of Leuconostocaceae. They are generally ovoid cocci often forming chains. Leuconostoc spp. are intrinsically resistant to vancomycin and are catalase-negative (which distinguishes them from staphylococci). All species within this genus are heterofermentative and are able to produce Leuconostocfrom sucrose. They generally form exopolysaccharide. [35]
Oenococcus
Oenococcus is a genus of Gram-positive bacteria, placed within the family Leuconostocaceae. The only species in the genus was Oenococcus oeni (which was known as Leuconostoc oeni until 1995). In 2006, the species Oenococcus kitaharae was identified. As its name implies, Oenococcus holds major importance in the field of oenology(the science and study of wine and winemaking), where it is the primary bacterium involved in completing the malolactic fermentation. [36]
Oenococcus kitaharae
O. kitaharae is a lactic acid bacterium (LAB) that was isolated from composting distilled shochu residue produced in Japan. This species represents only the second member of the genus Oenococcus to be identified. O. kitaharae has the ability to ferment maltose, citrate and malate and the ability to synthesize specific amino acids such as L-arginine and L-histidine unlike some O. Oeni. In addition to these metabolic differences, the O. kitaharae genome also encodes many proteins involved in defense against both bacteriophage (restriction-modification and CRISPR) and other microorganisms (bacteriocins), and has had its genome populated by at least two conjugative transposons, which is in contrast to currently available genome sequences of O. oeni which lack the vast majority of these defense proteins. It therefore appears that the genome of O. kitaharae has been shaped by its need to survive in a competitive growth environment that is vastly different from that encountered by O. oeni, where environmental stresses provide the greatest challenge to growth and reproduction. [37]
Sugar Utilization -
One of the defining biochemical differences between O. kitaharae and O. oeni that was noted in its original isolation was the ability of O. kitaharae to produce acid from maltose. This trait is rare in O. oeni, which is formally classified as maltose negative. By comparing available whole-genome annotations for O. oeni with O. kitaharae, it was possible to identify several genes associated with sugar utilization that are deferentially present across the species. Of these, at least four genes which are present in O. kitaharae, but absent in the O. oeni genomes, are predicted to be involved in the utilization of maltose, providing a direct genetic basis for this phenotype. In addition to genes predicted to be involved in the species-specific utilization of maltose, there are several ORFs predicted to be involved in the metabolism of trehalose, D-gluconate, D-ribose and fructose that are specifically present in O. kitaharae. While the assimilation of these sugars is often carried out by specific strains of O. oeni, this genotypic data agrees well with biochemical tests performed previously that indicated that O. kitaharae was able to utilize all of these various carbon sources. [38]
Oenococcus oeni
Oenococcus oeni(also know as Leuconostoc oeni) is a Genus of Gram-positive LAB, ellipsoidal to spherical in shape that is primarily used in Malolactic Fermentation. Oenococcus oeni is a facultative anaerobe. It is able to use oxygen for cellular respiration but can also gain energy through fermentation. It characteristically grows well in the environments of wine, being able to survive in acidic conditions below pH 3.0 and tolerant of ethanol levels above 10%. Optimal growth occurs on sugar and protein rich media. Cells tend to grow in chains or pairs. O. Oeni is heterofermentative and generally produces CO2, Ethanol, Acetate, and Diacetyl. [39]
O. oeni ferments sugars using both the hexose-monophosphate and phosphoketolase pathways using the enzymes Glucose-6-phosphate and xylulose-5-phosphoketolase to from D(-)-lactic acid, CO2 and ethanol in equal amounts when metabolising D-glucose. O.oeni can convert pentose phosphate to acetic acid in an oxygen dependant reaction which requires NADP. It cannot metabolize polysaccharides and alcohols.
O. oeni can decarboxylate L-malate to L(+)-lactate, but cannot use it as a sole source of carbon. It requires the amino acids Glutamic acid, valine, guanine, adenine, xanthine, uracil, riboflavin, folic acid, nicotinic acid, thiamine, biotine and pantothenic acid. There is some variation of amino acid requirement between strains. [40]
Althought O. oeni has primarily been used for Malolactic Fermentation, trials with the White Labs culture(only one reported on so far) has show lactic acid production without the presence of malic acid. James Sites reported souring within a week at 70°F. [41]
| Name | Mfg# |
|---|---|
| White Labs | Malolactic Culture |
| Wyeast | Malolactic Blend |
| CHR Hansen | Viniflora |
Weisella
See also:
Zymomonas mobilis
Zymomonas mobilis is a Gram negative, facultative anaerobic, non-sporulating, polarly-flagellated, rod-shaped bacterium. It is the only species found in the genus Zymomonas. It has notable bioethanol-producing capabilities, which surpass yeast in some aspects. It was originally isolated from alcoholic beverages like the African palm wine, the Mexican pulque, and also as a contaminant of cider and beer.[42]
About Health Concerns
Potential references
- Fermentation of cellobiose/glycosides https://www.ncbi.nlm.nih.gov/pmc/articles/PMC241500/ - https://onlinelibrary.wiley.com/doi/full/10.1002/jib.381?fbclid=IwAR1lBJsgLnyftqiYef9nJ5nNHAkaHCm64RFFLq-hrWapuSYLRghR8GOl22Y - http://beer.suregork.com/wp-content/uploads/2015/06/Poster-89.pdf - Bioflavoring by non-conventional yeasts in sequential beer fermentations http://www.sciencedirect.com/science/article/pii/S0740002017303763 and MTF comments - Bochman's published article on lactic acid producing yeast: http://www.sciencedirect.com/science/article/pii/S0740002017302952 - Performance of non-conventional yeasts in co-culture with brewers’ yeast for steering ethanol and aroma production (http://onlinelibrary.wiley.com/doi/10.1111/1751-7915.12717/epdf) - See Fig3B - Fugelsang K, Edwards C. Wine Microbiology. 1997. Available: http://link.springer.com/content/pdf/10.1007/978-0-387-33349-6.pdf - https://www.facebook.com/groups/MilkTheFunk/permalink/1336235339738010/?comment_id=1336277939733750&comment_tracking=%7B%22tn%22%3A%22R%22%7D - https://www.facebook.com/groups/MilkTheFunk/permalink/1337089182985959/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1346900285338182/ - http://www.sciencedirect.com/science/article/pii/S0963996916302332 - https://www.facebook.com/groups/MilkTheFunk/permalink/1366829093345301/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1365795896781954/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1380004022027808/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1284664904895054/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1400174630010747/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1420821137946096/ - http://www.sciencedirect.com/science/article/pii/S074000201630452X - https://www.facebook.com/groups/MilkTheFunk/permalink/1457271340967742/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1485339661494243/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1140282595999953/ - https://www.ncbi.nlm.nih.gov/pubmed/12102552 - https://www.facebook.com/groups/MilkTheFunk/permalink/1546044102090465/ - http://beer.suregork.com/?p=3860 - http://ijs.microbiologyresearch.org/content/journal/ijsem/10.1099/ijsem.0.001607 - https://www.facebook.com/groups/MilkTheFunk/permalink/1582089058485969/ - http://www.mbaa.com/publications/tq/tqPastIssues/2017/Pages/TQ-54-1-0215-01.aspx - http://biorxiv.org/content/early/2017/03/27/121103 - https://www.facebook.com/groups/MilkTheFunk/permalink/1640324282662446/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1659004047461136/ - https://www.facebook.com/groups/MilkTheFunk/permalink/1669790909715783/ - http://onlinelibrary.wiley.com/doi/10.1002/jib.381/full - http://www.asbcnet.org/publications/journal/vol/2017/Pages/ASBCJ-2017-2532-01.aspx - https://www.facebook.com/groups/MilkTheFunk/permalink/1680093658685508/ - http://onlinelibrary.wiley.com/doi/10.1002/yea.3146/abstract - https://mail.google.com/mail/u/1/?ui=2&ik=1b8e47c65b&view=att&th=15c548b66cda8ae6&attid=0.1&disp=safe&zw - https://www.facebook.com/groups/MilkTheFunk/permalink/1649825158379025/ - Pichia - http://www.sciencemag.org/news/2017/07/microbe-new-science-found-self-fermented-beer - Sherry Flor - https://www.facebook.com/groups/MilkTheFunk/permalink/1099692053392341/
See Also
Additional Articles on MTF Wiki
External Resources
- UC Davis list and characterization of many yeasts and molds found in winemaking.
- "Yeasts and Yeastlike Fungi", an overview of yeast groups and classification.
References
- ↑ Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. Cletus P Kurtzman. 2003. DOI: https://doi.org/10.1016/S1567-1356(03)00175-2.
- ↑ East Coast Yeast website. Wild Yeast / Brettanomyces / Lactic Bacteria. Retrieved 01/24/2018.
- ↑ 3.0 3.1 Fermmentos Labs Catalog. Retrieved 12/21/2017.
- ↑ "Launch of lactic-acid producing yeast – Sourvisiae®" lallemand Brewing blog. 09/12/2019. Retrieved 09/12/2019.
- ↑ Justin Amaral. Milk The Funk thread on pitching A2A as a primary or secondary pitch. 01/31/2018.
- ↑ Metschnikowia reukaufii. The Yeast Bay website. Retrieved 08/21/2019.
- ↑ 7.0 7.1 7.2 Wild Pitch Yeast catalog. Retrieved 1/2/2018.
- ↑ Dr. Matt Bochman. Milk The Funk thread on Saucy Brew Works use of lactic acid yeast. 01/12/2018.
- ↑ 9.0 9.1 9.2 Screening and Application of Cyberlindnera Yeasts to Produce a Fruity, Non-Alcoholic Beer. Konstantin Bellut, Maximilian Michel, Martin Zarnkow, Mathias Hutzler, Fritz Jacob, Jonas J. Atzler, Andrea Hoehnel, Kieran M. Lynch, and Elke K. Arendt. 2019. DOI: https://doi.org/10.3390/fermentation5040103.
- ↑ Konstantin Bellut. Milk The Funk Facebook group thread on Bellut et al. 2019 study on Cyberlindnera. 12/21/2019.
- ↑ Wikipedia. Debaryomyces. Retrieved 09/03/2015.
- ↑ Wikipedia. Debaryomyces hansenii. Retrieved 09/03/2015.
- ↑ 13.0 13.1 "The Original Flag Porter Story". Brewlab website. 01/20/2017. Retrieved 12/08/2017.
- ↑ 14.0 14.1 Exopolysaccharides from yeast: insight into optimal conditions for biosynthesis, chemical composition and functional properties - review. Iwona Gientka, Stanisław Błażejak, Stanisław Błażejak, Lidia Stasiak, Lidia Stasiak, Anna Chlebowska-Śmigiel, Anna Chlebowska-Śmigiel. 2015.
- ↑ 15.0 15.1 15.2 15.3 . Eureka Blog's Post on D. Hansenii, Retrieved 8/9/2017
- ↑ 16.0 16.1 16.2 . Production of ethanol and arabitol by Debaryomyces nepalensis: influence of process parameters. Himabindu Kumdam, Shweta Narayana Murthy and Sathyanarayana N Gummadi. 2013.
- ↑ 17.0 17.1 Saccharomyces cerevisiae, Non-Saccharomyces Yeasts and Lactic Acid Bacteria in Sequential Fermentations: Effect on Phenolics and Sensory Attributes of South African Syrah Wines. P.P. Minnaar, H.W. du Plessis, V. Paulsen, N. Ntushelo, N.P. Jolly, M. du Toit. 2017.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 Application of Non-Saccharomyces Yeasts Isolated from Kombucha in the Production of Alcohol-Free Beer. Konstantin Bellut, Maximilian Michel, Martin Zarnkow, Mathias Hutzler, Fritz Jacob, David P. De Schutter, Luk Daenen, Kieran M. Lynch, Emanuele Zannini, and Elke K. Arendt. 2018. DOI: https://doi.org/10.3390/fermentation4030066.
- ↑ 19.0 19.1 Lachancea thermotolerans as an alternative yeast for the production of beer. P.Domizio, J.F.House, C.M.L.Joseph, L.F.Bisson, and C.W.Bamforth. 2016.
- ↑ Bryan of Sui Generis Blog and Justin Amaral. Milk The Funk Facebook group post regarding the temperature range for L. thermotolerans. 10/22/2017.
- ↑ Justin Amaral and Bryan of Sui Generis blog. Milk The Funk post about the fermentation times for Lachancea thermotolerans. 11/6/2017.
- ↑ Ana Hranilovic, Joanna M. Gambetta, Leigh Schmidtke, Paul K. Boss, Paul R. Grbin, Isabelle Masneuf-Pomarede, Marina Bely, Warren Albertin & Vladimir Jiranek. 2018.
- ↑ Mrakia gelida in brewing process: An innovative production of low alcohol beer using a psychrophilic yeast strain. Giovanni De Francesco, Ciro Sannino, Valeria Sileoni, Ombretta Marconi, Sara Filippucci, Giorgia Tasselli, Benedetta Turchetti. 2018. DOI: https://doi.org/10.1016/j.fm.2018.06.018.
- ↑ . Wikipedia, Obtained 8/1/17
- ↑ Zach Taggart. Milk the Funk Facebook group post on EPS from yeast. 01/16/2019.
- ↑ 26.0 26.1 . Pichia k Info. Source: Eureka Brewing Blog.
- ↑ Genome Sequence of Pichia kudriavzevii M12, a Potential Producer of Bioethanol and Phytase.
- ↑ 28.0 28.1 . Identification of Pichia apotheca. Authors: Caiti Smukowski Heil, Joshua N. Burton, Ivan Liachko, Anne Friedrich, Noah A. Hanson, Cody L. Morris, Joseph Schacherer, Jay Shendure, James H. Thomas, Maitreya J. Dunham. 2017.
- ↑ Eukaryote Wiki. Retrieved 10/12/2017.
- ↑ S. pombe Micro Wiki. Retrieved 10/12/2017.
- ↑ Matt Bochman. Milk The Funk Facebook thread on S. pombe. 11/27/2017.
- ↑ 32.0 32.1 32.2 32.3 32.4 32.5 . Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Maximilian Michel, Jana Kopecká. 2016.
- ↑ Eureka's Blog post about T. Delbruecki, 02/10/2014 .
- ↑ Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. F.A. Cubillos, B. Gibson, N. Grijalva‐Vallejos, K. Krogerus, J. Nikulin. 2019. DOI: https://doi.org/10.1002/yea.3380.
- ↑ .
- ↑ .
- ↑ Identifcation of O. Kitaharae, Authors: Akihito Endo1, Sanae Okada1 10/1/2006 .
- ↑ . Functional Divergence in the Genus Oenococcus as Predicted by Genome Sequencing of the Newly-Described Species, Oenococcus kitaharae, Authors Anthony R. Borneman, Jane M. McCarthy, Paul J. Chambers, Eveline J. Bartowsky 01/3/2012 .
- ↑ "Oenococcus oeni". Microbe Wiki. Retrieved 07/20/2017.
- ↑ . UC Davis General Info on O. Oeni (No Date Given) .
- ↑ James Site. Milk The Funk Facebook group. 08/04/2015.
- ↑ Zymomonas mobilis .