Difference between revisions of "Hops"
(→Antimicrobial Properties) |
m |
||
| Line 73: | Line 73: | ||
Hops are known to have antimicrobial properties against Gram-positive bacteria. This includes bacteria that can be present in beer both as spoilage organisms and as intentionally added in sour and mixed fermentation beer such as ''[[Lactobacillus]]'' and ''[[Pediococcus]]''. Certain other bacteria found in beer such as ''Acetobacteraciae'' are Gram-negative and are not susceptible to the antimicrobial properties of hops <ref name="Hough_1957">[https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.1957.tb06267.x J. S. Hough, B.Sc, Ph.D., G. A. Howard, M.Sc., Ph.D., and C. A. Slater, Ph.D. 1957.]</ref><ref name="Macrae_1964">[https://onlinelibrary.wiley.com/doi/abs/10.1002/j.2050-0416.1964.tb02001.x SIGNIFICANCE OF THE USE OF HOPS IN REGARD TO THE BIOLOGICAL STABILITY OF BEER: I. REVIEW AND PRELIMINARY STUDIES. R. M. Macrae. 1964.]</ref>. Certain Gram-positive bacteria strains that have adapted to the brewing environment are known to be more resistant to the antimicrobial effects of hops. The antimicrobial effect is characterized as inhibiting the growth and lactic acid production of lactic acid bacteria, however, this does not always also include cell death as ''Lactobacillus'' that has been inhibited by hops can later be revived <ref name="Macrae_1964" />. Multiple mechanisms have been proposed to explain why hops are antimicrobially active. | Hops are known to have antimicrobial properties against Gram-positive bacteria. This includes bacteria that can be present in beer both as spoilage organisms and as intentionally added in sour and mixed fermentation beer such as ''[[Lactobacillus]]'' and ''[[Pediococcus]]''. Certain other bacteria found in beer such as ''Acetobacteraciae'' are Gram-negative and are not susceptible to the antimicrobial properties of hops <ref name="Hough_1957">[https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.1957.tb06267.x J. S. Hough, B.Sc, Ph.D., G. A. Howard, M.Sc., Ph.D., and C. A. Slater, Ph.D. 1957.]</ref><ref name="Macrae_1964">[https://onlinelibrary.wiley.com/doi/abs/10.1002/j.2050-0416.1964.tb02001.x SIGNIFICANCE OF THE USE OF HOPS IN REGARD TO THE BIOLOGICAL STABILITY OF BEER: I. REVIEW AND PRELIMINARY STUDIES. R. M. Macrae. 1964.]</ref>. Certain Gram-positive bacteria strains that have adapted to the brewing environment are known to be more resistant to the antimicrobial effects of hops. The antimicrobial effect is characterized as inhibiting the growth and lactic acid production of lactic acid bacteria, however, this does not always also include cell death as ''Lactobacillus'' that has been inhibited by hops can later be revived <ref name="Macrae_1964" />. Multiple mechanisms have been proposed to explain why hops are antimicrobially active. | ||
| − | One mechanism of the antimicrobial activity of hops is due to the role of iso-alpha alpha acids and possibly similar hop acids (such beta acids and oxidized hop acids) as ionophores, or compounds which can transport ions across cell membranes. While their antimicrobial properties are strong, alpha and beta acids in beer and wort and their effects on brewing are generally disregarded because they do not solubilize <ref name="Fernandez and Simpson, 1993"> [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.1993.tb02782.x/full Fernandez and Simpson (1993)] </ref><ref name="Sakamoto and Konings, 2003"> [http://www.sciencedirect.com/science/article/pii/S0168160503001533 Sakamoto and Konings (2003)]</ref>. The protonated iso-α-acid (the form of the acid with an associated H+ ion, an H+ ion is a proton) is the antimicrobially active form. This means that for a beer with a given iso-α-acid concentration, the antimicrobial effects will be stronger at lower pH values because a greater percentage of the acid will be protonated. Protonated iso-α-acids act against bacteria by crossing into the cell and dissociating (releasing H+ ions from the iso-α-acid and decreasing the pH within the cell), therefore disrupting the cellular proton gradient which is necessary for cells to function, before binding an equal charge in metal ions and crossing back out of the cell. Cells with resistance to hop bitter acids are better able to eject disassociated iso-α-acids from the cell and therefore preserve their proton gradients. The mechanism to expel iso-α-acids appears to be specific toward this type of compound rather than by a more general antimicrobial resistance mechanism such as multi-drug resistant bacteria possess <ref name="Sakamoto and Konings, 2003"/>. The anti-microbial power of iso-α-acids is pH dependent. At a higher pH (5.6) iso-α-acids begin to lose their anti-microbial properties, but at a typical beer pH (4.3) iso-α-acids inhibited a sample of 6 strains of ''L. brevis'' in one study <ref name="zhao_1027">[https://www.frontiersin.org/articles/10.3389/fmicb.2017.00239/full#B28 Heterogeneity between and within Strains of Lactobacillus brevis Exposed to Beer Compounds. Yu Zhao, Susanne Knøchel and Henrik Siegumfeldt. 2017. DOI: https://doi.org/10.3389/fmicb.2017.00239.]</ref>. Hop resistant bacteria cultured in the absence of hop acids can lose their resistance if grown in an environment without antibacterial hop compounds<ref name="Fernandez and Simpson, 1993"/> and some hop resistant microbes need to be acclimated to hop acids by growth in sub-limiting levels of antibacterial acids before they are able to resist higher levels <ref name="Sakamoto and Konings, 2003"/>. | + | One mechanism of the antimicrobial activity of hops is due to the role of iso-alpha alpha acids and possibly similar hop acids (such beta acids and oxidized hop acids) as ionophores, or compounds which can transport ions across cell membranes. While their antimicrobial properties are strong, alpha and beta acids in beer and wort and their effects on brewing are generally disregarded because they do not solubilize <ref name="Fernandez and Simpson, 1993"> [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.1993.tb02782.x/full Fernandez and Simpson (1993)] </ref><ref name="Sakamoto and Konings, 2003"> [http://www.sciencedirect.com/science/article/pii/S0168160503001533 Sakamoto and Konings (2003)]</ref>. The protonated iso-α-acid (the form of the acid with an associated H+ ion, an H+ ion is a proton) is the antimicrobially active form. This means that for a beer with a given iso-α-acid concentration, the antimicrobial effects will be stronger at lower pH values because a greater percentage of the acid will be protonated. Protonated iso-α-acids act against bacteria by crossing into the cell and dissociating (releasing H+ ions from the iso-α-acid and decreasing the pH within the cell), therefore disrupting the cellular proton gradient which is necessary for cells to function, before binding an equal charge in metal ions and crossing back out of the cell. Cells with resistance to hop bitter acids are better able to eject disassociated iso-α-acids from the cell and therefore preserve their proton gradients. The mechanism to expel iso-α-acids appears to be specific toward this type of compound rather than by a more general antimicrobial resistance mechanism such as multi-drug resistant bacteria possess <ref name="Sakamoto and Konings, 2003"/>. The anti-microbial power of iso-α-acids is pH dependent. At a higher pH (5.6) iso-α-acids begin to lose their anti-microbial properties, but at a typical beer pH (4.3) iso-α-acids inhibited a sample of 6 strains of ''L. brevis'' that exhibited a range of general hop tolerance in one study <ref name="zhao_1027">[https://www.frontiersin.org/articles/10.3389/fmicb.2017.00239/full#B28 Heterogeneity between and within Strains of Lactobacillus brevis Exposed to Beer Compounds. Yu Zhao, Susanne Knøchel and Henrik Siegumfeldt. 2017. DOI: https://doi.org/10.3389/fmicb.2017.00239.]</ref>. Hop resistant bacteria cultured in the absence of hop acids can lose their resistance if grown in an environment without antibacterial hop compounds<ref name="Fernandez and Simpson, 1993"/> and some hop resistant microbes need to be acclimated to hop acids by growth in sub-limiting levels of antibacterial acids before they are able to resist higher levels <ref name="Sakamoto and Konings, 2003"/>. |
Another antimicrobial mechanism resulting from oxidative stress has been attributed to both iso-α-acids and humulinic acids <ref name="Schurr et al, 2015"> [http://www.sciencedirect.com/science/article/pii/S0740002014002470 Schurr et al., (2015)] </ref>. Humulinic acids are either not bitter tasting or much less bitter than iso-α-acids but are similar in structure to and are formed from the degradation of iso-α-acids as well as during the aging of hops <ref>[https://www.sciencedirect.com/science/article/abs/pii/S0040402001981992 The absolute configuration of the isohumulones and the humulinic acids. D.De Keukeleire, M.Verzele. 1971. https://doi.org/10.1016/S0040-4020(01)98199-2.]</ref>. This oxidative stress-driven antimicrobial activity is due to the potential for oxidation-reduction (redox) reactions within bacterial cells between Mn2+ ions and these specific hop acids. The redox potential is due to different conditions inside (higher pH, higher Mn2+) and outside (lower pH, lower Mn2+) of the bacterial cell <ref name="Behr and Vogel, 2010"> [http://aem.asm.org/content/76/1/142.short Behr and Vogel, (2010)] </ref><ref name="Schurr et al, 2015"/>. Iso-α-acids or humulinic acids passing into the cell, form complexes with Mn2+ and transfer electrons out of the cell <ref name="Behr and Vogel, 2010"/>. By targeted molecular modifications [http://www.sciencedirect.com/science/article/pii/S0740002014002470 Schurr et al. (2015)] determined that the Mn oxidative stress-driven antimicrobial effect of iso-α-acids was more important than the antimicrobial effect of the ionophore proton transfer discussed above in the overall antimicrobial activity of hops. Thus, the antimicrobial effects of humulinic acids have been found to be even stronger than iso-alpha acids, suggesting that aged hops retain at least some antimicrobial properties at least partially from humulinic acids <ref name="Schurr et al, 2015"/>. | Another antimicrobial mechanism resulting from oxidative stress has been attributed to both iso-α-acids and humulinic acids <ref name="Schurr et al, 2015"> [http://www.sciencedirect.com/science/article/pii/S0740002014002470 Schurr et al., (2015)] </ref>. Humulinic acids are either not bitter tasting or much less bitter than iso-α-acids but are similar in structure to and are formed from the degradation of iso-α-acids as well as during the aging of hops <ref>[https://www.sciencedirect.com/science/article/abs/pii/S0040402001981992 The absolute configuration of the isohumulones and the humulinic acids. D.De Keukeleire, M.Verzele. 1971. https://doi.org/10.1016/S0040-4020(01)98199-2.]</ref>. This oxidative stress-driven antimicrobial activity is due to the potential for oxidation-reduction (redox) reactions within bacterial cells between Mn2+ ions and these specific hop acids. The redox potential is due to different conditions inside (higher pH, higher Mn2+) and outside (lower pH, lower Mn2+) of the bacterial cell <ref name="Behr and Vogel, 2010"> [http://aem.asm.org/content/76/1/142.short Behr and Vogel, (2010)] </ref><ref name="Schurr et al, 2015"/>. Iso-α-acids or humulinic acids passing into the cell, form complexes with Mn2+ and transfer electrons out of the cell <ref name="Behr and Vogel, 2010"/>. By targeted molecular modifications [http://www.sciencedirect.com/science/article/pii/S0740002014002470 Schurr et al. (2015)] determined that the Mn oxidative stress-driven antimicrobial effect of iso-α-acids was more important than the antimicrobial effect of the ionophore proton transfer discussed above in the overall antimicrobial activity of hops. Thus, the antimicrobial effects of humulinic acids have been found to be even stronger than iso-alpha acids, suggesting that aged hops retain at least some antimicrobial properties at least partially from humulinic acids <ref name="Schurr et al, 2015"/>. | ||
Revision as of 14:58, 16 December 2019
Hops are the flowers (also called seed cones or strobiles) of the female dioecious (meaning that they have separate male and female plants) plant Humulus lupulus [1], and are used in brewing for flavor as well as for antimicrobial properties. Although bitterness from boiling hops is generally not desired in sour beers, sour and funky brewers can use hops to help regulate lactic acid bacteria and control acid production to desired levels, especially in aged mixed-fermentation or spontaneous fermentation beers. Additionally, it may be argued that the earthy bitterness from aged hops is desired for lambic based styles (see Hops in lambic below). Potentially other mixed fermentation styles can benefit from some degree of bitterness either from aged or fresh hops such as saisons, farmhouse ales, and experimental styles. So while the mantra for sour beer is that "bitterness and sour don't work together", there are certainly exceptions to this rule. Brewers who are interested in rapid acid production using quick/kettle souring techniques such as wort souring may wish to limit or avoid hop use before acidifying so that sufficient acid is produced quickly.
Contents
Hop Composition
The main compounds of interest to brewers in hops are their bitter acids and oils contained in the yellow-colored lupulin glands. There are at least 250 significant aroma and flavor compounds found in hop acids and oils. Alpha acids account for roughly 2-17% of dried hops by mass, beta acids account for roughly 2-10%, and oils account for roughly 0.5-3%, though the exact percentages will vary depending on factors such as the hop varietal, growing region, harvest time, and growth conditions for the year. The rest of the weight of hops is contained in the leafy matter called Bract and is made up of 40-50% cellulose and lignin, 15% protein, 8-12% water (after drying), 8% minerals, 3-6% polyphenols and tannins, 1-5% lipids and fatty acids, 2% monosaccharides, and 2% pectin [2][3].
Acids
Alpha acids (also called "humulones" and abbreviated as "α-acids") in hops mostly consist of humulone, cohumulone, and adhumulone. The ratio of these individual acids to each other can vary based on hop variety much like total iso-α-acid percent, though generally the primary acids are humulone and cohumulone. Cohumulone has been identified by some researchers as a source of a more harsh bitterness, although similar research contradicts this statement [4]. While alpha acids are mostly insoluble in wort at typical brewing pH (alpha acids become much more soluble as the pH rises towards 5.9 to 7, which is not typical for wort production [5]), the isomerized alpha acids (also called isohumulones) which are formed during boiling are soluble. Isomerization leads to roughly a 70%/30% split between cis and trans iso-α-acids respectively, with cis iso-α-acids being more stable over time and more bitter[6]. Alpha acids themselves do not taste bitter, but isomerized alpha acids (iso-α-acids/isohumulones) contribute to the bitterness of beer and have antimicrobial properties. Isocohumulone is often cited as being more harshly bitter than the other iso-α-acids, but studies of taste perception of individual iso-α-acids have not agreed with this. However isocohumolone is slightly more soluble than the other acids and therefore a hop with a higher cohumulone composition may result in a beer with higher iso-α-acid for hops of equal iso-α-acid percent and use in brewing but different iso-α-acid breakdown[6]. Alpha acids are susceptible to oxidation and the alpha acid content of a hop will decrease with storage.
Beta Acids (lupulones) are similar in structure to alpha acids and have the analogous individual beta acids (lupulone, colupulone, adlupulone, prelupulone, and postlupulone [7]) to individual alpha acids. In their original form, beta acids do not contribute to the flavor of beer because they are not soluble in beer unless the pH of the boiling wort is significantly raised to around 7 pH (which is not typical in brewing conditions) and the original gravity is relatively low (2-8°P) [5]. They are also not able to isomerize during wort boiling. Beta acids do not become soluble in wort or beer unless they are chemically modified by a process such as oxidation [2], nor are they soluble in beer when dry hopping [8]. Oxidized beta acids are soluble and can contribute to bitterness in beer. Oxidized beta acids are discussed more under aged hops.
Isomerization of Alpha Acids
The isomerization of alpha acids into iso-alpha acids is mostly dependent on alpha acid content of the hops, time (to a certain extent), temperature, original gravity, hop rate (hop weight), and IBU saturation. Other variables also affect isomerization to a lesser extent such as pH and calcium concentration [9][10][11]. The higher the gravity of wort above 1.050 SG, the more proteins coagulate and drop iso-alpha acids out of solution (lower gravity worts are not affected by this). During fermentation, yeast cells can absorb iso-alpha acids, which results in further loss of iso-alpha acids in the finished beer [5]. Lower flocculating yeast strains tend to reduce the IBU in finished beer more than high flocculating yeast [11]. Significant isomerization of alpha acids can occur in water without sugar at all (temperatures around boiling are still required), which is relevant in the production of "hop tea" in traditional farmhouse brewing where hops are steeped in hot water for some time, and this is said to extract bitterness from the hops [10][12][13].
Malowicki and Shellhammer determined a calculation that predicts the isomerization rates of alpha acids into iso-alpha acids at different temperatures. Beginning at the boiling temperature of 100°C/212°F, which could be considered a rate of 100%, at 96°C/205°F the rate is 72%, and at 90°C/194°F the rate is 43%. This rate continues to drop significantly as the temperature of the wort decreases. At 82°C/180°F isomerization occurs at a rate of 17%. At a temperature of 50°C/122°F, the isomerization rate is at 1%, and finally 0% at 45°C/113°F. This fact has several impacts on brewing processes. For example, when brewing at higher altitudes where the boiling point of wort is less than 100°C/212°F, the isomerization of alpha acids into iso-alpha acids will be reduced to whatever the rate is at that lower temperature. "Hop stands" or "whirlpool additions" where hops are left in contact with hot wort that is less than boiling temperature will continue to isomerize alpha acids [14].
The hopping rate (weight) and IBU saturation have a drastic effect on IBU's. Aaron Justice reported a higher utilization when using Polaris hops (17.6% alpha acids) versus Tettnang hops (1.9% alpha acids). Two beers were brewed using enough of each of the hop varieties to target a calculated 40 IBU. However, the beer brewed with Polaris hops had 42.6 IBU and the beer brewed with Tettnang hops had only 28 IBU. It was hypothesized that the higher surface area of more hop matter reduces IBU's by binding to iso-alpha acids and possibly other compounds that register on the standardized IBU test. Justice also reported that more IBU pickup from whirlpooling was possible in beers without hops added in the boil, indicating that IBU saturation can limit IBU's, with a maximum IBU being around 100 [11].
The pH of the wort can also have a small effect on the isomerization of alpha acids to iso-alpha acids, although this variable is less significant than other variables such as alpha acid content, time (to a certain extent), temperature, and original gravity. Aaron Justice reported a trending slight rise in the conversion of alpha acids to iso-alpha acids when the boil pH was raised from 5.05 (~40% of aa's converted to iso-aa's) to 5.35 (~50% of aa's converted to iso-aa's) [11]. Bastgen et al. (2019) found that at a boil pH of 5.6, the percentage of iso-alpha acids increased by 32% by extending the boil from 60 minutes to 120 minutes. However, there was no increase at all in iso-alpha acids when the boil pH was 7, but a pH of 7 is not typical in the brewing process [5]. Time is thought to play a large role in isomerization, however, Justice reported that the majority of the IBU from iso-alpha acids in 60 minute additions and in whirlpool additions occurs within the first 10 minutes, with only a 12-30% increase after another 50 minutes of boiling/whirlpooling (higher gravity beers had more isomerization during the final 50 minutes while lower gravity beers had less isomerization during the final 50 minutes of boiling) [11].
See also:
Oils
There are three primary classes of oils in hops: hydrocarbons (~64% of the total oils), oxygenated compounds (~35% of the total oils), and sulfur compounds (≤1% of the total oils)[15]. Individual flavor and aroma active oils each have different thresholds, solubilities, and volatilities, and individual oils can have synergistic interactions with each other. The chemistry of hop oil taste perception is therefore very complicated and overall is not well understood. For example, only recently it has been shown that the amount of hop oils does not correlate to hop aroma intensity when dry hopping, but the composition of hop oils does [16]. While sulfur compounds make up only a very small fraction of the total oils, they have a significant impact on hop flavor [15].
Hydrocarbons, specifically terpenoids, make up the majority of hop oil. The majority of these terpenoids are myrcene, which characterizes the aroma of hops (although this compound does not carry over well into beer because it is hydrophobic), caryophyllene, and humulene. Most of these compounds are evaporated off by the brewing process, and others are metabolized into different compounds during fermentation [17]. Linalool (citrus, floral, fruity, tropical [16]) and geraniol (rose-like, musty, floral [16][18]) have been identified as the major compounds that contribute to beer flavor in hop varieties such as Cascade [19].
Hop oil contains a small percentage (~1%) of sulfur related compounds (thiols, sulfides, polysulfides, thioesters, thiopenes, and terpene derivatives). Although these levels are low, the flavor thresholds for these compounds also tends to be very low. Hydrogen sulfide can be released from these compounds during fermentation. Hops that have been treated with sulfur to prevent mildew growth (an older process that is generally no longer used) can result in increased sulfur compound such as sulfuric terpenes, and lend a garlic-like aroma in beer. Few sulfur compounds survive boiling, however late hopping and dry hopping preserves more sulfur compounds which can survive into the beer. Fermentation generally volatilizes sulfur compounds, and some volatilize almost completely during fermentation [20].
Thioesters are derived from an acid and a thiol. These include S-methyl hexanethioate and S-methyl heptanethioate and derivatives of these, which impart cooked cabbage, sulfuric, and soapy flavors, and their low flavor threshold can have an impact on finished beer. Sulfides and polysulfides found in hops includes dimethyl sulfide (DMS), dimethyl disulfide (DMSD), dimethyl trisulfide (DMST; cooked vegetable, onion). DMTS has been found in wide ranges in hops, from a few ppm to 1450 ppm, and has a very low flavor threshold (1 ppb). These compounds are volatilized during brewing and fermentation, and are generally only found in beers that are dry hopped [20].
Other thiol (organic sulfur) based compounds contribute to a pleasant aroma and flavor in beer, such as 4-mercapto-4-methyl-pentan-2-one (4MMP), which is found in high quantities in North American varieties such as Simcoe (highest amount), Summit, Apollo, Topaz, and Cascade hops, as well as varieties from Australia and New Zealand. The character of black currant, muscat-like aroma in beer brewed with these hops has been attributed to 4MMP. It is thought that 4MMP is only found in North American, Australian, and New Zealand hops and not European hops because European hops are often treated with copper ions, which has been shown to decrease the amount of 4MMP in hops. Interestingly, beers brewed with these hops showed a 33% increase in 4MMP after fermentation; it is thought that the precursor cysteine conjugate is responsible for the increase in 4MMP during fermentation [17]. The volatile thiols 3-sulfanyl-4-methylpentan-1-ol (3S4MP; grapefruit [21]), and 3-sulfanyl-4-methylpentyl acetate (3S4MPA; passionfruit, grapefruit [21]) have been identified in Nelson Sauvin hops as the compounds that give these hops their "wine-like, Sauvignon Blanc" character. Similar thiols have been described as the major contributors to the aroma of Sauvignon Blanc wines themselves: 3-sulfanylhexan-1-ol (3SH) and 4-methyl-4-sulfanyl-pentan-2-one (4MSP/4MMP) [19].
See also:
Characterizing Hop Flavor and Aroma
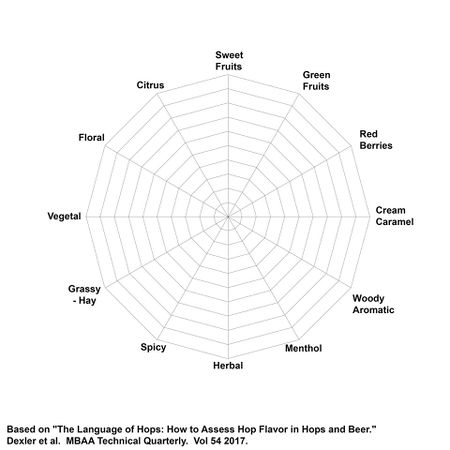
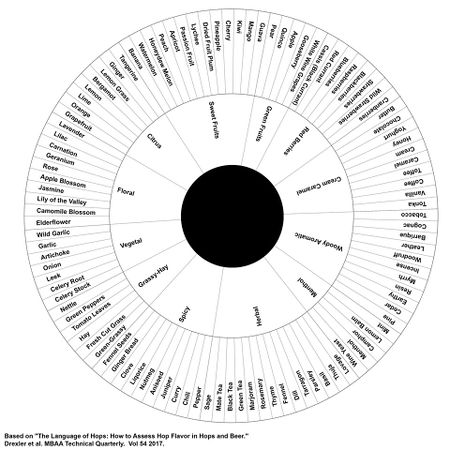
Hops provide a wide array of aromas and flavors to beer. These flavors and aromas are variety and crop dependent. Hop farmers often provide their own hop flavor and aroma descriptors independently of each other, but attempts to standardize these descriptors have been made as far back as 1756, and as recently as 1978. More recently, Drexler et al. (2017) worked with a perfumer to establish 12 major categories of hop flavor descriptors. Each major category contains more specific descriptors. These descriptors can be measured on a 0-10 scale, and a spider graph can be drawn to represent them. Drexler et al. (2017) proposed that even though expensive gas chromatography is available for hops which measures specific compounds, sensory analysis is still the best way to quantify how different varieties of hops actually smell and taste in beer [22].
The proposed categories, example hop variety, and the specific descriptors by Drexler et al. (2017) are as follows [22]:
- Floral (ex: Ella): Elderflower, Chamomile Blossom, Lily of the Valley, Jasmine, Apple Blossom, Rose, Geranium, Carnation, Lilac, Lavender
- Citrus (ex: Mandarina Bavaria): Grapefruit, Orange, Lime, Lemon, Bergamot, Lemon Grass, Ginger, Tangerine
- Sweet Fruits (ex: Mosaic®): Banana, Watermelon, Honeydew Melon, Peach, Apricot, Passion Fruit, Lychee, Dried Fruit Plum, Pineapple, Cherry, Kiwi, Mango, Guava
- Green Fruits (ex: Hallertau Blanc): Pear, Quince, Apple, Gooseberry, White Wine Grapes
- Red Berries (ex: Monroe): Cassis (Black Currant), Red Currant, Blueberries, Raspberries, Blackberries, Strawberries, Wild Strawberries, Cranberries
- Cream Caramel (ex: Triskel): Butter, Chocolate, Yoghurt, Honey, Cream, Caramel, Toffee, Coffee, Vanilla, Tonka
- Woody Aromatic (ex: Relax): Tobacco, Cognac, Barrique, Leather, Woodruff, Incense, Myrrh, Resin, Earthy, Cedar, Pine
- Menthol (ex: Polaris): Mint, Lemon Balm, Camphor, Menthol, Wine Yeast
- Herbal (ex: Columbus): Lovage, Thuja, Basil, Parsley, Tarragon, Dill, Fennel, Thyme, Rosemary, Marjoram, Green Tea, Black Tea, Mate Tea, Sage
- Spicy (ex: Saazer): Pepper, Chili, Curry, Juniper, Aniseed, Nutmeg, Liqorice, Clove, Ginger Bread, Fennel Seeds
- Grassy-Hay (ex: Herkules): Green-Grassy, Fresh Cut Grass, Hay, Tomato Leaves, Green Peppers, Nettle
- Vegetal (ex: Summit®): Celery Stock, Celery Root, Leek, Onion, Artichoke, Garlic, Wild Garlic
See also:
- "Influence of Hops and Yeast", Alicia Muñoz Insa presentation at the 2015 Belgian Brewing Conference.
- MBAA Podcast interviews Georg Drexler about his study.
- Experimental Brewing Podcast talks about the Drexler et al. study (starts at ~36 minutes in).
- YCH hop variety database.
- Brulosophy's "The Hop Chronicles", an attempt to characterize hop flavor and aroma.
- Hopsteiner presentation on specific blends of hops that match the flavor profile of another hop.
Antimicrobial Properties
Hops are known to have antimicrobial properties against Gram-positive bacteria. This includes bacteria that can be present in beer both as spoilage organisms and as intentionally added in sour and mixed fermentation beer such as Lactobacillus and Pediococcus. Certain other bacteria found in beer such as Acetobacteraciae are Gram-negative and are not susceptible to the antimicrobial properties of hops [23][24]. Certain Gram-positive bacteria strains that have adapted to the brewing environment are known to be more resistant to the antimicrobial effects of hops. The antimicrobial effect is characterized as inhibiting the growth and lactic acid production of lactic acid bacteria, however, this does not always also include cell death as Lactobacillus that has been inhibited by hops can later be revived [24]. Multiple mechanisms have been proposed to explain why hops are antimicrobially active.
One mechanism of the antimicrobial activity of hops is due to the role of iso-alpha alpha acids and possibly similar hop acids (such beta acids and oxidized hop acids) as ionophores, or compounds which can transport ions across cell membranes. While their antimicrobial properties are strong, alpha and beta acids in beer and wort and their effects on brewing are generally disregarded because they do not solubilize [25][26]. The protonated iso-α-acid (the form of the acid with an associated H+ ion, an H+ ion is a proton) is the antimicrobially active form. This means that for a beer with a given iso-α-acid concentration, the antimicrobial effects will be stronger at lower pH values because a greater percentage of the acid will be protonated. Protonated iso-α-acids act against bacteria by crossing into the cell and dissociating (releasing H+ ions from the iso-α-acid and decreasing the pH within the cell), therefore disrupting the cellular proton gradient which is necessary for cells to function, before binding an equal charge in metal ions and crossing back out of the cell. Cells with resistance to hop bitter acids are better able to eject disassociated iso-α-acids from the cell and therefore preserve their proton gradients. The mechanism to expel iso-α-acids appears to be specific toward this type of compound rather than by a more general antimicrobial resistance mechanism such as multi-drug resistant bacteria possess [26]. The anti-microbial power of iso-α-acids is pH dependent. At a higher pH (5.6) iso-α-acids begin to lose their anti-microbial properties, but at a typical beer pH (4.3) iso-α-acids inhibited a sample of 6 strains of L. brevis that exhibited a range of general hop tolerance in one study [27]. Hop resistant bacteria cultured in the absence of hop acids can lose their resistance if grown in an environment without antibacterial hop compounds[25] and some hop resistant microbes need to be acclimated to hop acids by growth in sub-limiting levels of antibacterial acids before they are able to resist higher levels [26].
Another antimicrobial mechanism resulting from oxidative stress has been attributed to both iso-α-acids and humulinic acids [28]. Humulinic acids are either not bitter tasting or much less bitter than iso-α-acids but are similar in structure to and are formed from the degradation of iso-α-acids as well as during the aging of hops [29]. This oxidative stress-driven antimicrobial activity is due to the potential for oxidation-reduction (redox) reactions within bacterial cells between Mn2+ ions and these specific hop acids. The redox potential is due to different conditions inside (higher pH, higher Mn2+) and outside (lower pH, lower Mn2+) of the bacterial cell [30][28]. Iso-α-acids or humulinic acids passing into the cell, form complexes with Mn2+ and transfer electrons out of the cell [30]. By targeted molecular modifications Schurr et al. (2015) determined that the Mn oxidative stress-driven antimicrobial effect of iso-α-acids was more important than the antimicrobial effect of the ionophore proton transfer discussed above in the overall antimicrobial activity of hops. Thus, the antimicrobial effects of humulinic acids have been found to be even stronger than iso-alpha acids, suggesting that aged hops retain at least some antimicrobial properties at least partially from humulinic acids [28].
The oxidized forms of hop acids have been shown to have a limited inhibitory effect on Gram-positive bacteria. This might explain the anecdotal experiences of some brewers that have tried using aged hops that were high alpha varieties and produced beer that wasn't sour. Stevens et al. (1961) reported that a strain of Lactobacillus that was cultured from infected beer was inhibited by alpha acids at 40 ppm, beta acids at 10 ppm, iso-alpha acids at 160 ppm, and oxidized beta acids (cohulupone) at 200 ppm. So, while the oxidized beta acids had the least inhibitory power, a high concentration was still inhibitory [31]. Oxidized alpha acids (humulinones) have only been tested for antibacterial properties at a concentration of 50 ppm or less. At 50 ppm, oxidized alpha acids were not able to inhibit two strains of Lactobacillus that were isolated from infected beer, as reported by Hough et al. (1957) [23]. See oxidized hop acids for more information on oxidized hop acids.
Dry hopping has also been demonstrated to inhibit lactic acid bacteria. See Dry Hopping below.
- See also Lactobacillus hop tolerance.
Bacterial Resistance to Hop Compounds
Due to the multiple mechanisms for hop antimicrobial activity, multiple resistance mechanisms are necessary for a Gram-positive bacterial cell to successfully be hop-tolerant[30]. Hop resistance of bacteria will vary by species as well as within a species with individual strains. The environment in which strains are cultured and maintained may also influence their hop tolerance. It is possible for a small subpopulation of individual cells that have a higher tolerance to hops to eventually dominate the overall population [27]. The hop tolerance of lactic acid bacteria strains decreases when they are cultured in hop-free environments and strains cultured in media with increasing concentrations of hop compounds show an increase in hop tolerance[26]. The stability of hop resistance, or the rate at which it is lost when bacteria are cultured in unhopped wort, varies by strain. It can take up to 1 year for maximum loss of hop resistance, suggesting that in some strains have a relatively stable hop resistance[26]. Because of this intra-species variability and dependence on how the strains were cultured, it is difficult to give specific advice about the hop-tolerance of a wide range of strains offered from different sources. As a general rule, some common lactic acid bacteria species used in sour beer and found as beer spoilage organisms like Lactobacillus brevis, Lactobacillus lindneri and Pediococcus delbrueckii have some resistance to hops[26]. Brewers seeking to make acidic beers with higher doses of hops may wish to seek out one of these species. Some hop-tolerant species benefit from pre-culturing in media with below-limiting concentrations of compounds before being used in more highly hopped wort or beer[32].
See also Pediococcus hop resistance and Lactobacillus hop tolerance.
Hop Derived Compounds In Beer and Biotransformations
The flavor and aroma compounds found in leaf/pellet hops are different than the hop-derived flavor and aroma compounds found in finished beer (other than in the case of dry hopping). The brewing process (particularly boiling), and fermentation greatly affect the composition of flavor and aroma compounds that are found in beer. For example, boiling wort and hops isomerizes non-bitter alpha acids into bitter iso-alpha acids. During the boiling of the wort, many compounds found in hops are evaporated, such as many of the various sulfur compounds found in hops. The terpene hydrocarbons which make up most of the hop oil content in hops (myrecene, humulene, and caryophyllene) are completely removed by fermentation. It is believed that these terpene hydrocarbons stick to the yeast cells and fall out of solution during fermentation [33].
A "biotransformation" is any change in a chemical's structure that is initiated by a living organism [34]. It has been hypothesized that biotransformations of some kind are taking place in beer during fermentation and explain changes to hop compounds during fermentation and beer storage. Some carbonyl compounds found in hops (citral, geranial, nerol, citronellal, and methyl ketones) can be used as a food source by yeast during fermentation. Cyclic ethers such as linalool oxides, karahana ether, hop ether, and rose oxide (aroma of roses [35]), increase after fermentation and have been identified as secondary metabolites produced by yeast during metabolism from hop derived precursors. Esters found in hops can be converted into ethyl esters by yeast during fermentation; for example, geranyl esters found in Cascade hops can be hydrolyzed into geraniol (flowery). The terpenoid citronellol (citrus and floral [36]) can be esterified by yeast fermentation into citronellyl acetate (fresh, rosy, fruity odor reminiscent of geranium oil [37]). Yeast strains differ in their ability to convert these compounds. For example, one study found that lager yeast was able to form acetate esters of geraniol and citronellol, but ale yeast was not [33].
Terpenes and terpenoids (monoterpene alcohols) can also be transformed by fermentation. Studies have found that geraniol and nerol can transform into linalool by a strain of S. cerevisiae, as well as nerol and linalool into alpha-terpineol, which can then be further transformed to terpin. Geraniol can also be converted into citronellol, and the content of geraniol and citronellol can be increased in finished beer by increasing the initial content of geraniol, which is found in higher quantities in some varieties of hops (Citra, for example). Linalool, nerol, and alpha-terpineol gradually decrease during fermentation and aging (perhaps being transformed into ethers, which is a class of organic compound that contains an oxygen atom connected to two alkyl or aryl groups), while nerol and citronellol gradually increase. Geraniol also decreases during fermentation, but not as drastically as linalool. It has been hypothesized that the bioconversion of geraniol into citronellol could be by means of glycosidic activity (although another study found that glycosidic activity in S. cerevisiae is not very strong). Post-fermentation dry hopping preserves linalool and alpha-terpineol, and limits citronellol to trace levels [33].
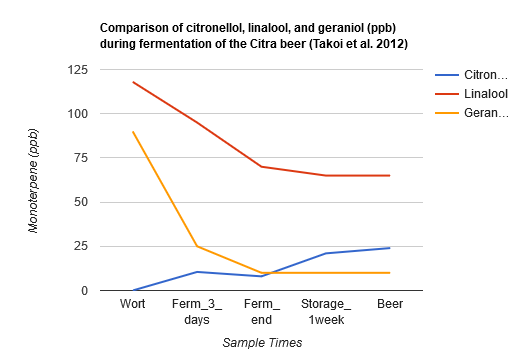
Takoi et al. (2012) used Citra hops with a high content of geraniol added late in the boil, and reported a steep decline on geraniol during the first three days of fermentation with a lager yeast strain. Linalool had a gradual decline but ended up at higher levels than geraniol in the finished beer. Citronellol had a sharp increase during the first three days of fermentation and then remained at a stable level until the end of fermentation. However, after storing the beer at 15°C (59°F) for 1 week, the amount of citronellol more than doubled. This indicated that active fermentation may not be required for the transformation of geraniol into citronellol (the yeast was filtered before packaging the finished beer, after a storage time of 6-8 days at 13–15°C and then at 0°C for 2–3 weeks). Interestingly, Takoi et al. (2012) also showed that coriander seeds, which also have high levels of linalool and geraniol, have a nearly exact same effect on beer, with a beer made with 0.5 g/L of coriander seed resulting in 20 ppb of citronellol and a beer made with 0.75 g/L of coriander seed resulting in 30 ppb of citronellol. The Citra beer had a citrus and "green" aroma, while the coriander beers had a very floral aroma with a slight citrus impression. They also conducted a sensory experiment with different levels of geraniol and citronellol added to linalool to see if small amounts of these would affect the flavor of a large dosage of linalool, and the results confirmed that small increases of geraniol and citronellol increased flowery and fruity flavors even in the presence of high dosages of linalool [38]. The data for the Citra beer is shown below:
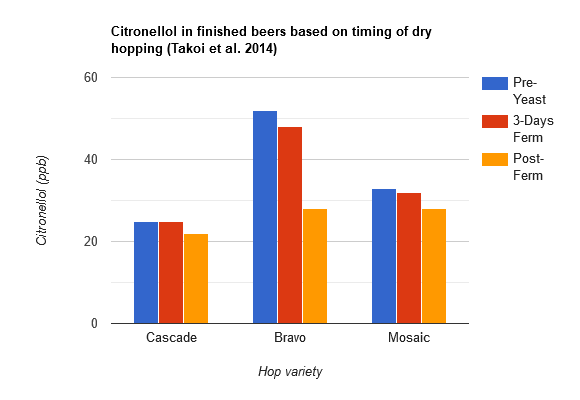
Takoi et al. (2014) continued their research into monoterpene biotransformations. They determined that some varieties of hops have higher concentrations of geraniol (floral flavor) than others, which when used in beer, can lead to higher citronellol levels (citrus flavor) in beer that wasn't present in the hops or wort. They found that while traditional German hops such as Saaz and New Zealand hops contain very little geraniol, American hops such as Bravo, Citra, Cascade, Mt. Hood, Mosaic, Chinook, Apollo, Amarillo, and others contain relatively large amounts of geraniol, with significant variations from different crop years. In this study, they measured the amount of linalool, geraniol, and citronellol in beers that were dry hopped at different time points: before yeast was added (labeled "pre-yeast" in the table below, and represents something similar to whirlpool hop additions), 3 days after yeast was added, and at the end of fermentation. For each of these timings, they tested three different hop varieties that contained high levels of geraniol: Cascade, Bravo, and Mosaic. Overall, the amount of linalool in the finished beers wasn't affected by the timing of the dry hop. The amount of citronellol was also not affected by the timing of the dry hop except for the Bravo hops where the post-fermentation hopping resulted in about half the amount of citronellol than it did for the pre-fermentation and 3-day fermentation dry hop timings (see the bar graph based on the data from Takoi et al. 2014 below). The timing of the dry hop had the largest effect on geraniol: the earlier the dry hop, the less geraniol was present in the finished beer for all three hop varieties, with hops added pre-fermentation producing the lowest amount of geraniol and hops added post-fermentation producing the most geraniol in the finished beers. As in their previous study, citronellol increased during the first three days of fermentation, remained relatively stable for the rest of fermentation, and then increased again during storage. Geraniol dropped significantly during the first three days of fermentation in the case of the pre-yeast and 3-day dry hop timing and increased slightly during storage. This data indicates that while earlier dry hopping reduces geraniol, only certain varieties of hops have an increase in citronellol depending on the dry hop timing. It's been suggested that the transformation of geraniol to citronellol involves unknown mechanisms that are relatively complex, particularly because the rate of the disappearance of geraniol does not map onto the rate of increase in citronellol, and when post-fermentation dry hopping there is a high amount of free geraniol but not a corresponding increase in citronellol during storage [39].
See also this table which shows the higher geraniol levels from post-fermentation dry hopping (labeled "Timing 1") versus lower geraniol levels from pre-yeast (labeled "Timing 2") and 3-day fermentation dry hopping (labeled "Timing 3").
Other yeast species can also convert monoterpenes. For example, a strain of Kluyveromyces lactis was found to reduce geraniol to citronellol. This strain and a strain of Torulaspora delbrueckii produced linalool from both geraniol and nerol, and could also form geraniol from nerol [40]. Many species of Debaryomyces, Kluyveromyces, and Pichia were found to transform geraniol into linalool, and nerol into linalool and alpha-terpineol [41].
Sulfur-based compounds known as thiols have also been shown to be produced by yeast fermentation from hop derived precursors (suspected to be S-glutathione). So far, science has found that these include the volatile thiols 3-sulfanyl-4-methylpentan-1-ol (3S4MP; grapefruit) and 3-sulfanyl-4-methylpentyl acetate (3S4MPA; passionfruit, grapefruit). These thiols were found in beers dry hopped separately with Amarillo, Hallertau Blanc, and Mosaic hop varieties. The amounts of these two thiols were higher than expected based on the content of these thiols in the hops alone [21]. See also this MTF thread speculating on how Brettanomyces might produce thiols.
In general, different yeast strains have a large impact on how hops are perceived in the final beer, including both perceived bitterness and flavors. For example, POF+ (phenolic positive) strains of Saccharomyces cerevisiae tends to mask the hop-derived aromas in dry hopped beers [16]. A beer hopped with the Tradition hop variety produced fruit flavors when fermented with Abbaye ale yeast, and woody/spicy flavors when fermented with US-05. When the beer was brewed with Citra hops, with US-05 the beer had sweet fruits/citrus flavors and more bitterness, but when fermented with the Abbaye ale strain the beer had a more one dimensional sweet fruit/floral flavor and less bitterness [42].
See also:
Glycosides
Hops contain glycosides, which are flavor compounds that are bound to a sugar molecule. In their bound form, glycosides are flavorless. Studies on hop compounds elude to the possibility of compounds being produced by the glycosidic activity of S. cerevisiae, however direct evidence of glucosidic activity in S. cerevisiae is lacking. Daenen (2008) reviewed the glycosidic activity of many strains of S. cerevisiae, and found that only a few strains expressed any real glucosidic activity and none that exhibited exo-beta-glucosidase which would be required to break glycosidic bonds in the beer/wort. Daenen did find that enzymatic activity from some strains of Brettanomyces can efficiently release these bound compounds and release their flavor and aromatic potential [33]. Beta-glucosidase enzyme can also be added to beer to enhance the breakdown of glycosides and intensify hop-derived flavors and aromas. For example, one study showed an increase in citrus, orange, grapefruit, and tropical pineapple in a Cascade dry hopped beer that had beta-glucosidase enzymes added to it [43]. There is also some evidence to support that there is higher glucosidase activity in seeded hops, which are generally not used in the brewing industry [44].
Much of the work on hop derived glycosides has been done using hop oils, and might not apply to whole cones or pellet hops. Sharp et al. (2017) found that when using pure beta-glucosidase extract on beer hopped with whole leaf hops that the amount of increased monoterpenes such as linalool, terpineol, citronellol, nerol, and geraniol is small and insignificant. The fatty alcohol 1-octanol (waxy, green, citrus, orange, aldehydic, fruity [45]) was the only measured flavor compound that was increased significantly [46]. The alcohol octanol can be esterified into octyl acetate, which is a classically "citrusy" aroma, so perhaps certain yeasts can create this ester during mid-fermentation hopping [47].
See Glycosides for more information on glycosides.
Lightstruck
See Aging and Storage.
Aged Hops
Aging hops leads to oxidation of acids and oils. Generally, brewers seek to avoid this to preserve the aromatic and bittering properties of their hops by freezing them and storing them in vacuum sealed packaging (oxygen exposure is by far the larger factor for hop degradation, followed by ambient room temperatures, which is significant because hops are often not stored in vacuum sealed packaging). However, some beer styles, including lambic and historical styles, make extensive use of aged hops. Aged hops still retain some antimicrobial properties at least partially from the formation of humulinic acids (see Antimicrobial Properties of Hops), and they can be used for microbial inhibition. In addition to their antimicrobial activity, aged hops contribute important flavor and aroma compounds and precursors to beer, while not contributing much of a strong bitterness from iso-alpha acids. These flavor descriptors often include herbal, tea-like, Earth-like, and a more dull bitterness. Low amounts of Isovaleric Acid might also contribute to the complexity of a beer that has been brewed with aged hops (although the presence of isovaleric acid in aged hops is considered temporary, and will eventually age out of hops that are aged). Historically, some brewers had issues keeping mildew from growing on aged hops that are aged in higher humidity areas (sulfur was used to combat mildew, which often gave the beer a sulfur, rotten egg aroma) [48][49].
In lambic brewing, the term aged hops refers to hops (usually Noble varieties such as Tettnang, Saaz, Target, and Hallertau) which have been aged for 3-5 years in non-refrigerated conditions, and in burlap sacks or some other oxygen permeable bag [50][51]. It should be noted that the term "aged hops" can also refer to any sort of hop aging (especially in scientific literature), including short-term hop aging (1-6 months, for example) at refrigerated or non-refrigerated temperatures, and in oxygen-rich or vacuum sealed packaging. Much of the information below references hops that have been aged in warm conditions for shorter time periods than what hops are aged for in lambic brewing. The additional aging of hops that are used in lambic brewing or similar beers might have different effects than what has been studied in hops that are aged for shorter periods of time.
For techniques and usage amounts of aged hops, see Aged Hops in Lambic.
Aging Hops
Typically, only low alpha acid hops are used (high alpha acid hops may lead to more hop character and higher inhibition of lactic acid bacteria than desired possibly due to oxidized acids). The hops are typically bound in burlap sacks/paper bags or something similar that allows for exposure to oxygen, and then they are left to age in preferably low humidity conditions at room temperatures to warm temperatures (warmer temperatures will encourage faster aging). Changes in the environment such as temperature shifts are not a concern; for example Jester King Brewery in Austin Texas ages hops in a horse barn. The hops should are traditionally aged for 2+ years. Monitor for mold growth during this time, and discard any hops that show visible signs of mold growth. Some brewers prefer to age the hops until the cheesy character (Isovaleric Acid) is gone, while other brewers do not mind the presence of this cheesy character (for example, some lambic beers display isovaleric acid character even after packaging). While hop leaves are generally preferred over pelletized hops, if aging pellets, it has been advised to break the pellets up so that the entirety of the hop material is exposed to the air.
Freshly harvested hops (also called "wet hops") should not be aged. Freshly harvested hops should be dried first, as is normal for hop processing, before aging in order to prevent mold growth (see this article from Michigan State University on measuring moisture levels and this AHA article on drying hops for home growers).
See also:
- Joshua Martinez MTF thread on using a rotary composter to pulverize pellet hops for aging.
- MTF thread on general tips on aging hops.
Chemistry and Characteristics
Acids
During aging, both alpha and beta acids oxidize and degrade with warmer temperatures and more oxygen exposure having a greater impact. The generally accepted theory is that oxygen interacts directly with hop acids. This event is called "autooxidation". An alternative theory to this is that oxygen indirectly oxidizes acids by first oxidizing the hop oils and turning them into pro-oxidants, which then oxidize the hop acids which are mixed in with the oils within the lupulin glands [2]. The oxidation of hop acids corresponds with an increase in the Hop Storage Index (HSI), which is a practical way of measuring the overall freshness of hops. As the oxidation of hop oils rises, the measured HSI number on a lot of hops increases [52][53]. These oxidized compounds lead to a higher amount of non-alpha-acid bitterness compounds in aged hops and have a remarkable effect on the bitterness of the beer. The bitterness from oxidized hop compounds has been described as more earthy, harsh, and astringent than the sharper, cleaner bitterness from iso-alpha acids [54].
Aging hops while exposed to oxygen develops a cheesy aroma due to isovaleric acid, isobutyric acid, and 2-methylbutyric acid. These acids are produced by the oxidative cleavage of acyl side chains of the hop resins [55]. These cheesy oxidation compounds can be esterified to form wine-like and fruity tasting compounds (see Esters below and Aging and Storage) [15].
Storage conditions and variety play a large role in how acid content in hops changes over time. Beta acids are generally more resistant to oxidation than alpha acids. A study by Mikyška and Krofta (2012) found that after 12 months of storage at 20°C in open air, pellet hops lost 64-88% of their alpha acid content and 51-83% of the beta acid content, with the beta acids dropping off more significantly after 6 months (alpha acid content declined steadily throughout the aging period). These amounts varied with different Czech hop varieties (Saaz, Sládek, Premiant, and Agnus), and beta acids degraded slower than alpha acids as seen below [54] (percentages listed below are how much percent was lost):
| Storage | Oil | Saaz (pellet) [54] | Sládek (pellet) [54] | Premiant (pellet) [54] | Agnus (pellet) [54] | Saaz (leaf) [56] | Vital (leaf) [56] | Pure Beta Acid [56] |
|---|---|---|---|---|---|---|---|---|
| Open air at 20°C for 12 months | ||||||||
| Alpha acids | -80% | -88.3% | -64.3% | -78.2% | ||||
| Beta acids | -60.5% | -83% | -53.7% | -51% | -50% | -77.5% | -99% | |
| Vacuum sealed at 20°C for 12 months | ||||||||
| Alpha acids | -20.6% | -24.9% | -22.2% | -21.7% | ||||
| Beta acids | -2.7% | -1.7% | -2.1% | -1.2% | ||||
| Vacuum sealed at 2°C for 12 months | ||||||||
| Alpha acids | -1.1% | -5.5% | -0.3% | -1.4% | ||||
| Beta acids | -1.7% | -2.3% | -0.4% | -0.5% | ||||
Oxidized alpha acids (humulinones) are similar in taste perception to iso-α-acids, but have been described as less bitter (an average of about 66% as bitter on a 1 to 1 basis). The quality of the bitterness from oxidized alpha acids has been described in one study as "smoother and less lingering" than iso-alpha acids; this was attributed to humulinones being more polar than iso-alpha acids and therefore do not stick or linger on the tongue as long as iso-alpha acids [15][53]. While the taste threshold of iso-alpha acids is 5-6 mg/L in light lager, the threshold for humulinones has been measured to be 8 mg/L in light lager (note that this is an average; tasters vary widely in how much bitterness they perceived from different bitter compounds) [2]. Humulinone content increases in hops after being pelletized (whole leaf hops have less humulinones). In fresh pellet hops that have a relatively low humulinone content, the humulinones contribute little to the bitterness of the beer when boiled, however when dry hopped they readily dissolve into the beer and have a significant impact on the beer's bitterness. With heavy dry hopping, the humulinones also decrease iso-alpha acid content of beer with more than about 25 IBU's, but not in beer with less than about 20 IBU. The decrease in iso-alpha acids and perceived bitterness/IBU is partially made up for the bitterness of the humulinones themselves (humulinones are picked up in IBU measurements with a spectrophotometer and as such it has been suggested that IBU's be measured more accurately with HPLC). In beers with less than 20 IBU, high dry hopping rates greatly increase the bitterness/IBU due to the bitter humulinones. The rate of humulinone formation is limiting, meaning that humulinone formation occurs rapidly during hop pelletization, and the concentration peaks during this time (researchers found that further exposure to air did not increase humulinone content). Scientists believe that this is because when whole leaf hops are baled, only 20% of lupulin glands are broken, whereas when they are pelletized 100% of the lupulin glands are broken. The exact mechanism by which alpha acids are converted to humulinones is not known [53]. Humulinone content in long-aged hops (1+ years) has not been studied.
Oxidized beta acids produce some compounds that also contribute to the perception of bitterness, specifically hulupones. Unlike humulinones which form relatively quickly from the oxidation of alpha acids, hulupones form at a much slower rate [7]. Also unlike humulinones, they survive boiling and fermentation. While some sensory analysis of beers containing oxidized beta acids describes the resulting bitterness as "harsh and clinging", another analysis by Krafta et al (2013) described the bitterness of oxidized beta acids in beer when added in their pure form at the beginning of the boil as "pleasant and not lingering". The more degradation of beta acids into oxidized beta acids that occurs in hops, the more bitter beers brewed with these hops will be [56]. Two other compounds other than hulupones have been identified as being produced by the oxidation of beta acids, epoxycohulupone and epoxyhulupone. Their effect on beer flavor is not yet known; however, it is believed that hulupones have a greater impact on beer flavor and bitterness than these compounds [7].
The bitterness of hulupones has received some debate among researchers. In 1973, a researcher found that hulupones are about 50% as bitter as iso-alpha acids. Briggs et al stated the complete opposite, and that hulupones are twice as bitter as iso-alpha acids. More recent studies using modern analysis techniques found that on a weight for weight basis, hulupones are 35-40% as bitter as iso-alpha acids in one study, and another found that they were 84% (+/- 10%) as bitter as iso-alpha acids (note that this is an average; tasters vary widely in how much bitterness they perceived from different bitter compounds) [57][2][55]. While the taste threshold of iso-alpha acids is 5-6 mg/L in light lager, the threshold for hulupones has been measured to be 7-8 mg/L in light lager [2].
Both humulinones and hulupones have been identified as forming due to the oxidation of hop acids. However, other researchers have reported that both of these bitter compounds formed during the boiling of hops, and another during the storage and aging of beer. In all cases, the amounts of the compounds directly correlated with the amount of hops used [2].
Other compounds have been associated with the oxidation of beta acids and are extracted during wort boiling. These are described as giving a long-lasting, lingering bitterness on the palate. They include hydroxytricyclo-lupulone, dehydrotricyklolupulone, and hydroperoxytricyklolupulone [58].
The overall effect of oxidized compounds in aged hops has been shown by Val Peacock, a former scientist at Anheuser-Busch. Peacock stored hops at four different temperatures for 18 months. His data showed that although the alpha acid content in the hops and the iso-alpha acid content in the beers brewed with them decreased the older the hops were stored, the measured IBU of the different beers was about the same. This is because the oxidized acids in hops show up in the same spectrum as iso-alpha acids when using the ASBC method of measuring IBUs with a spectrophotometer [59]. This data is shown below. Caleb Buck's experiment seen below supports this. Although it has not been shown that oxidized alpha and/or beta acids can inhibit lactic acid bacteria, if they do, then this might help explain reports [60] of using aged hops that originally had a high alpha acid content retaining a strong inhibitory effect towards lactic acid bacteria.
| Storage Temperature [59] | Alpha Acid in Hops | Iso-Alpha Acids in Beer | Beer IBUs |
|---|---|---|---|
| -15°F | 3.2% | 19.8 ppm | 13.5 |
| 25°F | 2.91% | 18.1 ppm | 12.0 |
| 45°F | 1.71% | 14.4 ppm | 13.5 |
| 70°F | 0.41% | 2.9 ppm | 11.0 |
Oils
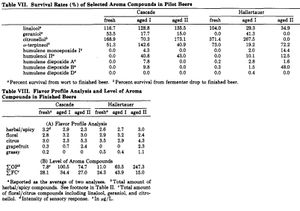
Hop oils also generally degrade over time, however, their degradation rates are more complex. Lam et al. (1986) found that aging both cascade and North American grown Hallertauer Mittelfrueh resulted in an increase in grapefruit-like character, although the compound that caused this was not identified. In the case of Cascade the intensity of this flavor correlated with the age of the hops [52]. In the Hallertauer hops, aging resulted in an increase in a spicy/herbal character [52], which is in agreement with reports of oxidized sesquiterpenes (specifically humulenol II, humulene diepoxides, caryophyllene, and to a lesser extent humulene monoepoxides and alpha-humulene) contributing a spicy/herbal flavor to beer [61][54]. Many of the oils followed in the Lam et al. (1986) study which increased during a short accelerated aging period (2 weeks at 90°F) then decreased during extended aging (60 additional days at 90°F). The cascade hops lost more of the fruity/citrusy hop oils (myrecene, linalool, and geranial) than Hallertauer, suggesting that different strains of hops can withstand aging better than others. The concentration of hop oils are affected by the brewing process and fermentation (see the table) [52]. Another study found that beta-ionone (classified as a ketone, and characterized as "floral" and "woody" [62]) increased in beers brewed with hops that were aged for 30 days at 40°C versus beers brewed with aged hops [63].
A recent study at the Shellhammer lab looked at how trained panelists and consumers perceived a lager beer dry hopped with slightly oxidized Hallertau Mittelfrüh hops (exposed to oxygen once, then stored at 38°C for two weeks) versus highly oxidized (daily exposure to oxygen and stored at 38°C for two weeks). They found that the trained panelists detected more characteristics that are associated with noble hops; e.g. more woody, earthy, and herbal characteristics in the lager beers dry hopped with oxidized hops. They also found the oxidized hopped beers to be more bitter (probably due to oxidized alpha and beta acids). Consumers were not statistically able to tell the difference. The study determined that oxidized hops might serve to provide nuanced increases in noble hop character [64].
- "Increasing Bitterness By Dry Hopping", article by Scott Janish on oxidized alpha acids.
- Hulupones - oxidized beta acids.
Polyphenols
Polyphenols, including polyphenol flavanoids, also degrade in hops as they age. However, storage conditions have less of an impact on the degradation of polyphenols compared to alpha and beta acids. Mikyška and Krofta (2012) found that regardless of how the hops were stored polyphenols started to decay after about 6 months and after 12 months aged hops lost about 30-40% of polyphenols and 20-30% of flavanoids [54].
Esters
During fermentation, it is believed that esters are produced by yeast metabolism from hop compounds such as alpha acids, beta acids, polyphenols, and hydrocarbons because they are not found in unhopped beer or in hops themselves. These esters include ethyl 2-methylpropanoate (citrus, pineapple, sweetness), ethyl 2-methylbutanoate (citrus, apple-like), ethyl 3-methylbutanoate (citrus, sweetness, apple-like), 2-phenylethyl 3-methylbutanoate (floral, minty), and 4-(4-hydroxyphenyl)-2-butanone (citrus, raspberry) [65]. Kishimoto et al. found that some beer esters were increased when using unidentified pellet hops (described in the study only as "a bitter variety of 11.5% alpha acid") that were aged for 30 days at 40°C versus using fresh pellet hops that were stored cold (4°C). Specifically, in the beers that used the aged hops, they found a significant increase in citrus esters (ethyl 2-methylbutanoate, ethyl 3-methylbutanoate, and 4-(4-hydroxyphenyl)-2-butanone), and a decrease in "green, hop-pellet-like, and resinous" compounds such as myrcene and (Z)-3-hexen-1-ol in the beers made from aged hops. The beers brewed with aged hops were described as more citrusy, while the beers brewed with fresh pellet hops were described as more "hop pellet-like", resinous, floral, and "green". The authors speculated that since these esters were not present in beers brewed without hops that they were derived from the humulone and lupulone oils in the hops during yeast fermentation [63].
Oxidation of alpha acids, beta acids, and iso-alpha acids can lead to the formation of isovaleric acid, isobutyric acid, 2-methylbutyric acid, and 3-methylbutyric acid. These compounds can also be produced by fermentation without hops, but in smaller amounts, with ale strains producing more than lager strains. These acids can then become esterified during fermentation and beer aging to produce the compounds ethyl isovalerate, ethyl 2-methyl butyrate, and ethyl 3-methyl butyrate, which have been suspected to be partially responsible for wine-like character in aged beers and have been measured to form in beer after about 3 months of storage at room temperature [66][67]. See also Esters in Aging Beer.
Thiols
Kishimoto et al. found an increase in the thiol 3-methyl-2-butene-1-thiol (MBT) in beers that were brewed with unidentified pellet hops (described in the study only as "a bitter variety of 11.5% alpha acid") that were aged for 30 days at 40°C versus using fresh pellet hops that were stored cold (4°C). Interestingly, this thiol was higher in beers where the aged hops were added to the boil rather than when they were added after the wort was cooled. The authors were not able to determine whether or not the MBT was derived from yeast fermentation, or from boiling the hops, but aging the hops increased the precursors for MBT [63]. MBT has been described as the thiol that produces the "skunky" aroma in lightstruck beer [68].
See Also
Aged Hop Suppliers
- Mainiacal Yeast aged hops (small lots for homebrewers).
-
Hops Direct "Choice Debittered/Aged Hops" (Leaf - Cascade). - Hops Direct "Choice Debittered/Aged Hops" (Pellet - Columbus).
- Freshhops "Lambic Hops" (Leaf - Willamette)".
- Yakima Valley Hops "Lambic / Aged Hops" (Pellet).
- Farmhouse Brewing Supply "Lambic Hop Blend" (Pellet - Blend of varieties that are aged for ~5 years and then pelletized [69]).
- The Malt Miller (UK).
- Brew Store UK (Leaf - Fuggles).
- Brew Store UK (Leaf - Hallertau).
- Northwest Hop Farms (BC, Canada).
- YCH offers 44 lb bags of aged hops; contact for more information.
- BSG sometimes offers aged pellet hops for commercial brewers; contact for details.
- Ted from Hop Heaven on eBay sells 8 oz and 1 lb bags of aged pellet and leaf hops. See reviews on MTF.
- Humlegårdens (Sweden); several varieties of aged whole leaf hops, 100 gram quantities.
- Humlegårdens (Sweden); several varieties of aged whole leaf hops, 100 gram quantities.
Cryo Hops® and Debittered/"American Noble" Hops
YCH Hops has patented a process of extracting hop oils from hops using a proprietary cryogenic separation process that is claimed to preserve all of the components of each hop fraction. They also distribute the leftover hop material as "Debittered Leaf" or "American Noble". These Debittered Leaf products have been reported to taste like low flavor/aroma/alpha versions of their original variety (for example, debittered Mosaic tastes like lower alpha Mosaic). They reportedly do not have the same character has aged "lambic" hops [70].
Techniques
Kettle and Mash Hopping
Kettle hopping sour beers can be a difficult thing for the new sour beer brewer. The usage of hops generally inhibits most lactic acid bacteria species, however there are many exceptions to this. Lactic acid bacteria can have a range of hop tolerance, with species such as Lactobacillus acetotolerans that tolerated Goose Island's Bourbon County Stout at 60 IBU [71]. Some breweries report that their house lactic acid bacteria can tolerate IBU ranges up to 10-20 IBU. White Labs claims that their L. delbuekii (WLP677) is tolerant of up to 20 IBU, however, most Lactobacillus cultures from yeast labs are not hop tolerant [72]. See the Lactobacillus culture charts and hop tolerance for more information.
For both mixed fermentation sour beers and kettle sour beers, hops are often not used at all. In the case of kettle sours, sometimes brewers opt to add hops after the wort has been soured (see Wort Souring). Commercial brewers in the USA must by law use 7.5 pounds of hops for 100 barrels of beer [73] (malt beverages without hops can still be approved by the FDA instead of the TTB; contact the TTB for guidance [74]). Since there is no US regulation for when the hops must be added, mash hopping might be a considered technique for commercial breweries in the US and in other parts of the world where hops are a requirement for beer (mash hopping retains only about 30% of the IBU that a 60 minute boiling addition does [75]). In historical German Berliner Weisse brewing, mash hopping or boiling hops during the decoction were also typical techniques (see Berliner Weisse historical brewing). Another historical technique for adding hops to beer is to add a hop tea (hops boiled in water), for example in historical raw ale brewing [76]. For lactic acid cultures that are hop tolerant, hops can be used as a way to inhibit the amount of acid produced by them if the brewer desires this. Another advantage of using at least some hops in the kettle is that various compounds from hops contribute to head retention, and using a small amount of hops in the kettle (and perhaps dry hopping) can greatly assist with head retention in sour beers.
A popular technique for 100% Brettanomyces Fermentation is to use a typical IPA recipe. Hops do not inhibit Brettanomyces yeast. Some of the fruity characteristics of Brettanomyces can complement the fruity character of hops such as Citra, Amarillo, and Galaxy. For beers that are fermented with just S. cerevisiae and Brettanomyces but not lactic acid bacteria (such as some American farmhouse ales), Old World and noble hops are often used as well as North American and New Zealand/Australian citrusy hops, depending on what flavor and aroma profile the brewer is intending.
See also:
Whirlpool Hopping
On commercial systems, adding hops during the whirlpool has become a common technique. The idea is that hopping during the whirlpool will decrease the amount of isomerization of alpha acids in the final beer, while providing flavor and aroma from the hops.
Aaron Justice reported that a considerable amount of isomerization occurs on both a 150 BBL system (75-90 minutes of total steeping time), a 50 BBL system (65-80 minutes of total steeping time), and a 5 BBL pilot system (35-40 minutes of total steeping time). Justice reported an average of 30% utilization (the amount of iso-alpha acids from the potential alpha acids), with a 12.2% deviation. The deviations were based on the gravity of the wort and the amount of hops added before the whirlpool. With lower gravity worts and worts with less kettle additions, a high increase in utilization was observed. Most of the IBU's were gained within the first 10 minutes of the whirlpool, with only very small increases in IBU (~3 IBU) after 10. This data indicates that the total whirlpool steeping time and thus brewhouse size does not necessarily have a large impact in the amount of isomerization that occurs during commercial whirlpooling. The temperature of the whirlpool was not reported [11].
Dry Hopping
Brewers have had positive and interesting results dry-hopping sour and funky beer. Often fresh American or New Zealand varieties that complement fruit flavors are chosen, however, other varieties have been used as well, including English and German hops. Just as in dry hopping normal beers, dry hopping sour/funky should be done after the beer has matured. Dry hopping for around 1-3 days before packaging the beer is adequate for extraction, depending on whether or not the beer is recirculated or agitated (agitation of the beer while on contact with the dry hops attains full extraction in 24 hours) [77]. Hopping rates generally range from 0.5-1 ounces per 1 gallon of beer (1-2 pounds per bbl or 3.7-7.5 grams per liter) to achieve hop-forward flavors, although lesser rates can be used to achieve a more subtle character (see the threads below) [78].
Dry hopping can contribute to bitterness in beer through oxidized alpha acids and oxidized beta acids. Oxidized alpha acids can also reduce iso-alpha acids in beers that begin with more than 25 IBU from iso-alpha acids, potentially reducing percieved bitterness after dry hopping (see Oxidized Alpha Acids above). Some alpha acids will also dissolve into the beer, which are estimated as being 10% as bitter as iso-alpha acids. Dry hopping also has a linear impact on the pH of beer regardless of the starting IBU or pH: the pH rises by 0.14 per pound of hop pellets per barrel of beer in a beer that started with a pH of 4.2 (~0.5 ounces per gallon or 3.7 grams per liter) [53][15]. This rise in pH might be less in more acidic beers that are dry hopped since pH is a logarithmic scale. Dry hopping can also reduce head retention in beers, although this is variety dependent (one study found that dry hopping with Eureka and Apollo hops increased head retention, while dry hopping with Bravo, Centennial, and Cascade decreased head retention). Extended dry hopping times (after 3 days) can also reduce head retention [8].
See also:
- The Rare Barrel reports on an anecdote that dry hopping in a less sour beer extracts better hop aroma, and Brettanomyces preserves the character.
- MTF thread by Dave Janssen on experiences with doing long-term dry hopping with noble hops in sour beers. See also Dave's research on a 1911 saison that was dry hopped long term.
- MTF thread on the possibility/mechanism of dry hopping contributing to the "funky" character of sour beers.
- MTF thread on dry hopping with aged hops.
- MTF thread from Benedikt Koch on using aged hops in a natural cider, inspired by Revel Cider.
Inhibiting Lactic Acid Bacteria
Dry hopping inhibits Gram-positive bacteria such as Lactobacillus. Humulinic acids have been found to greatly inhibit bacteria (see Antimicrobial Properties). Other compounds such as non-isomerized alpha acids, oxidized hop acids, or the small amount of isomerization of alpha acids that happens in beer at room temperature [79], could contribute to inhibiting lactic acid bacteria. See antimicrobial properties above. See also reported data below.
- Caroline Whalen Taggart's data point on the effects of dry hopping on L. plantarum (GoodBelly). No hops finalized at a pH of 3.53, and the dry hopped version finalized at a pH of 4.35. She used around 4 grams per gallon of 10-15 AA hops [80].
- Per Buer's experiment on the effects of dry hopping on Lactobacillus:
The Freshening Power of the Hop (Hop Creep)
Also known as "dry hop creep", it was first discovered in 1893 by Brown and Morris that dry hopping increases the ABV of beers and dries them out. It was proposed that the likely cause is the release of glycolytic enzymes that break down starches into sugars which viable yeast can then ferment. Brewers normally aim to control the final alcohol percentage in a beer through brewhouse operations rather than postfermentation dilutions with lower/higher alcohol beers or water. This approach to brewing is called "brewing to final gravity." Due to the need to have a predictable ABV for government regulatory reasons, unexpected fermentation is, therefore, a concern for many breweries [81]. Hop creep can also result in additional attenuation and higher carbonation after packaging, as well as diacetyl production.
Historically, there have been two studies published on the phenomenon of hops releasing glycolytic enzymes that break down starches during dry hopping: Brown and Morris (1893) and Janicki et al. (1941). More recently, several researchers and brewers have revisited this phenomenon. Brown and Morris (1893) discovered that hops could break down maltodextrin, but failed to identify the enzymes from the hop plant material and hypothesized (probably incorrectly) that tannins were inhibiting the enzymes. Janicki et al. (1941) came to similar conclusions regarding the enzymes and tannin inhibitors, and they also concluded that the enzyme activity was independent of hop variety, geography, age, storage conditions, pH values between 4.1 and 4.8, and that one or more additional unknown factors were at play [81].
More recent studies have shown that there is a difference in this enzymatic power between different hop varieties. Cibaka et al. (2017) reported an increase in ABV when dry hopping with Amarillo and Sorachi Ace hops, but not when dry hopping with Citra or Hallertau Blanc. Interestingly, they also found that Mosaic hops resulted in the opposite effect; the Mosaic dry hopped beer dropped from 4% ABV to 3.6% ABV. It was hypothesized (possibly incorrectly) that Mosaic hops might release some sort of unidentified molecule that inhibits yeast fermentation/growth or viability. Cibaka et al. (2017) also demonstrated that late kettle additions might work to combat dry hop creep; the beer dry hopped with 2 g/L of Sorachi Ace finished at 5.1% ABV, while a beer that received a late kettle addition of 2 g/L of Sorachi Ace in addition to 2 g/L of Sorachi Ace dry hop finished at 4.3% ABV (the control with no dry hop finished at 4.0% ABV) [81].
Kirkendall et al. (2018) found that hop varieties also have a varying ability to ferment dextrins. They reported the following ABV increases when dry hopped in a pale ale at one pound per barrel: Centennial hops (+0.27%), Citra (+0.12%), Simcoe (+0.33%), Cascade (+0.49%) and Amarillo (+0.49%). Prolonged contact with Centennial hops (42 days) increased the ABV even more so and resulted in a nearly 1% ABV increase. Rousing the hops into suspension hastened the increase in ABV compared to samples that were left still. From their results, it appears as though contact with hops during dry hopping continues the breakdown of starches and dextrins into fermentable sugars. They also concluded that dry hopping at a temperature that is too cold for the yeast strain in the beer to ferment resulted in no change in ABV. They compared the enzymatic activity of Centennial hops that were stored at -20°C versus room temperature storage and found that there was no significant difference, indicating that the unidentified enzymes are relatively stable [81].
Kirkpatrick and Shellhammer (2018) found that the enzymes responsible for the conversion of dextrins into sugars include amyloglucosidase (removes glucose from non-reducing ends of α-1,4 and branching α-1,6 linkages, with a preference for α-1,4 linkages and longer chain oligosaccharides), α-amylase (hydrolyzes randomly along glucopolysaccharides to produce maltose, maltotriose, maltopentaose, and maltohexaose products from amylose as well as maltose, glucose, and branched dextrins from amylopectin), β-amylase (saccharifiying enzyme, cleaving maltose in small amounts from nonreducing ends of glucopolysaccharides, and to a minor extent, maltotriose), and limit dextrinase (debranches limit dextrins at α-1,6 linkages, producing linear α-1,4 chains which can further be degraded by the combined action of amylases). They were able to successfully extract them from Cascade pellet hops using commercially available assays (enzyme specific para-nitrophenyl blocked oligosaccharide substrates). The amount of α and β-amylase found in Cascade hops was well below that of malted barley, but within the range reported in other plant leaves. These enzymes are denatured by high temperatures, and as such would be denatured when boiling hops. They reported a similar increase in ABV of 1.3% after 40 days when dry hopping a beer with Cascade hops (and a decrease of 1.9°P) at a rate of 10 g/L. They also found that the hops contained glucose and a small amount of fructose, which accounted for a sugar increase of 0.02−0.03 °P per gram of hops. More studies on whether or not the amount of dry hopping has a large effect needs to be done, and whether or not warmer temperatures speed up the enzymatic breakdown of dextrins, and the authors hypothesized that the rate of dextrin break down could be slowed by dry hopping at lower temperatures [82].
The exposure time of the beer to the dry hop material played a significant role in the breakdown of dextrins. Most of the breakdown of dextrins occurs within 5 days (+0.7% ABV), but continued slowly up until at least 40 days (+1.3%). They also tested removing the hops via centrifuge and storing the beer at 10°C or 20°C. Their results suggested that the effect of the enzymatic breakdown of dextrins by hops appears to only be active when in contact with the hops and that once the beer is removed then this breakdown of dextrins stops. The authors suggest that to avoid as much breakdown of starches and over-attenuation from dry hops as possible, brewers can limit the amount of time sits on the hops and reduce the temperature, however, it is also important to consider how this might impact the product's flavor and careful measures should be taken to balance the over-attenuation problem and overall beer quality [82]. After removing the beer from the hops, a second diacetyl rest has been suggested as a way to clean up any diacetyl or off-flavors that the yeast produces from the additional fermentation during dry hopping [83]. Other recommended solutions to avoiding hop creep is pasteurizing, filtering, or cold crashing out the yeast before dry hopping, storing the beer cold so that the yeast remains inactive, reducing dry hopping amounts, and dry hopping before fermentation is finished [84].
See also:
- Kirkpatrick and Shellhammer poster at the 2017 ASBC Annual Meeting.
- CDR BeerLab® experiment showing dry hop creep effects.
- Article on Rockstar Brewer Academy on how dry hop creep can cause diacetyl.
- Dry Hop Creep presentation by Caolan Vaughan in Australia.
- CBC presentation on dry hop creep by Allagash Brewing Company.
- Methods for avoiding hop creep and diacetyl production from hop creep explained by Nick Zeigler ("Hop and Brew School" podcast).
- MBAA podcast episode 98 on dry hop creep.
Aged Hops in Lambic
Modern lambic traditionally uses aged hops at a moderate rate to help limit and select for microbes and regulate acid production. Modern Lambic brewers cite rates in the range of roughly 450 grams of hops per Hl of finished beer [85] (~43 min in) (see also the notes pertaining hopping rates on the Cantillon page), with some brewers possibly going above this range. The age of hops used depends on the producer and their preferences/stock. Cantillon uses hops that are roughly 3 years old[86], while 3 Fonteinen reports using hops that are over 10 years old[87] (~48 minutes in). Jester King reported using 0.66 - 0.75 pounds of whole leaf aged hops per BBL (0.34-0.39 ounces per gallon) in their spontaneously fermented ales [88] (~31:00 mins in). Lambic brewers either add their hops while still collecting wort, sometime before the wort comes to a boil[89] (also known as "first wort hopping"), or shortly after boil is reached[87] (~48 min in). The hops are then boiled with the wort for essentially the full length of the boil [90][91]. The resulting lambic beers are often surprisingly bitter, especially when young. Historically, there is some evidence that lambic brewers used a combination of aged hops and fresh dried hops. Not all aged hops are the same; different varieties/sources result in different levels of residual alpha/beta acids (probably not zero), oxidized acids, IBU's, perceived bitterness, and inhibition of lactic acid bacteria. Varieties with high acids and hop oils probably have more residual acids and oils, and aging times/conditions may not be ideal enough to completely age high alpha/beta/oil hop varieties. Therefore, it is impossible to give a blanket statement on how much aged hops to use given a specific lot of aged hops. Andrew Holzhauer from Funk Factory Geuzeria suggests tasting aged hops for bitterness and adjusting the amount of hops depending on how bitter they taste [92], while James Howat from Black Project suggests making a small batch and having the wort/beer analyzed for IBU's and adjusting accordingly.
For example, homebrewer Caleb Buck performed an experiment comparing two different hopping rates for spontaneously fermented beer at home using whole leaf aged hops that were independently tested to have 0.5% alpha acids and 0.2% beta acids and were obtained from Hops Direct in Junuary 2016 [93]. The two rates tested were 0.3 ounces of aged hops per gallon and 0.6 ounces per gallon. Samples of the two worts were sent to Sweetwater Science Labs to perform IBU analysis using the ASBC standard IBU test. Interestingly, the results were 72 IBU and 127 IBU respectively. The unexpectedly high IBU might be due to the variety of aged hop, as well as oxidized hop acids showing up in the standard IBU test (see Peacock's data here that showed that aged and fresh dried hops produce a similar IBU). After about 7 months, one of the 0.3oz/gal batches got down to a pH of 3.6, a second batch at 0.3oz/gal got to a pH of 4, while the 0.6oz/gal batches remained within a pH of 4.2 - 4.3. From this experiment, Caleb will attempt using only 0.15 oz/gal of aged hops which should be closer to 30 IBU and so that more acidity can be achieved. James Howat from Black Project Spontaneous Ales suggests making sample wort with the hops that will be used for a larger batch and sending that sample off for IBU testing in order to more easily achieve the desired IBU's. More detail can be found on Caleb Buck's collected data on cooling rates, acidity from hopping rates, and other collected data over a multi-year, multi-batch experiment and Caleb's interview on this experiment on BasicBrewing Radio.
See also:
- "Lambic characteristics - FG and IBUs in Geuze" on Hors Categorie blog.
- "Hops in spontaneous fermentation" on Hors Categorie blog.
- MTF discussion on methods of aging leaf and pellet hops.
- MTF discussion on the general benefits of using aged hops in sour beers.
- MTF discussion on IBU's, alpha acids, oxidized bittering compounds, etc. might play a role in aged pellet hops.
- Lambic
- Spontaneous Fermentation
- Gueuze and Lambic Character
Historic hopping in lambic and other mixed-fermentation beer
While modern lambic uses aged hops almost exclusively, it was common for historic lambic to blend both aged and fresh hops[94]. The exact ratio of fresh to aged hops changed over time and could vary depending on the harvest (poor hop years may have relied more heavily on aged hops while years of good harvests would make more use hops of the recent harvest). In addition to the difference in hop age between modern and historic lambic, hopping rates also differ significantly between modern and aged hops. It is important to note that the quality of these hops are certainly different from modern hops, and that hop origin could have a significant influence on suggested hopping rates [95] (see the hopping rate table and notes regarding hop origin conversion factors from historical texts). While hop quality would have improved moving to the modern day while hopping rates were dropping, there is mention in historic lambic literature of lambic in the late 1800s being more bitter than lambic from the mid-1900s (and, subsequently, similar to historic saison in the increased hop presence in a mixed-fermentation beer)[94]
Historical documents dealing with Belgian brewing show a steady progression from high doses of fresh hops in lambic to the sort of hop composition and origin that are in use today. In 1851 Lacambre mentions rates for Belgian hops of 760-860 g/Hl and specifically highlights the use of young hops. Belgian brewing scientist Henri Van Laer recommended a hopping rate of 700-800 g/Hl in 1890, roughly in agreement with Lacambre though slightly lower. In the early 1900s, citing information from 1896, Le Petit Journal du Brasseur mentions a hopping rate of 540 g/Hl using a mix of Belgian and Bavarian hops and a split of 2/3 young, 1/3 old in good years (and 50/50 in bad years). In 1928 Le Petit Journal du Brasseur recommends a larger proportion of aged hops (2/3 aged, 1/3 fresh) and rates of 600g/Hl of Belgian hops[94]. Considering the difference in strength in German and Belgian hops[95], this fits with a stable or decreasing hopping rate from that given in the early 1900s. In 1937 exclusive use of aged hops is recommended, though as noted in 1946, year old hops may be preferable to hops that were aged longer in poor conditions[94]. Also in the 1940s Le Petit Journal du Brasseur recommends hopping rates of 400-500 g/Hl, roughly in agreement with modern times, and notes that the lambic of this time was softer than historic lambic[94].
(In Progress) Lambics aren't the only historic mixed-fermentation beer to make use of aged hops. Though the specific mention of aged hops for saison and bieres de garde does not seem to be the norm, aged hops were used at times, such as when more acidity was desired. These hops were also more likely to be used toward the beginning of the brewing season in months like October where the current harvest may have been considered too fresh for proper use. Notes: Give some discussion of hopping saison and bieres de garde. See hopping grisette table for some hopping rates, PJB, etc.
James Howat of Black Project Spontaneous Ales uses 0.5 ounces of aged hops per gallon of beer for spontaneously fermented beers brewed using traditional lambic techniques [96].
See also:
- MTF discussion on using aged hops in other styles of beers, including historical references and tips on producing lambic-like character from aged hops and commercial cultures.
- MTF discussion on leaving hops beer fermented with Brettanomyces long term; inspired by historical English brewing methods.
See Also
Additional Articles on MTF Wiki
External Resources
- Virginia Tech Virginia Cooperative Extension "Hops" webpage: growing and agriculture resources.
- "The Hop Plant Dissected" by Lisa Meoli, 2015.
- "Hoppy Sour Beers", Dec 2016 BYO article by Michael Tonsmeire.
- "Dry Hopping Effect on Bitterness and IBU Testing" by Scott Janish.
- YCH hop variety database.
- Per Buer's Video Demonstration of how dry hopping inhibits Lactobacillus.
- Blog Article on Brett and Glycosides by Cy Wood.
- "How hops prevent infection", by Lars Garshol.
- "Unlocking Hop and Fruit Flavors from Glycosides", HBT article by Dennis L Waldron.
- Beer Legends Hop Varieties - gives vital statistics on hops including acid content, physical cone characteristics, storage and growth details, and oil content.
- TTB Hop Requirements for USA commercial breweries.
- Blog article on hops in spontaneous fermentation by Dave Janssen
- Cohumulone ranges by hop variety.
- "CRISPR Yeast and Hop Compounds" by Dr. Matt Humbard; a critical response of yeast being modified to make two hop compounds.
- Summary of a Shellhammer presentation on dry hop saturation, bitterness from dry hopping, and hop creep (Pat's Pints blog, Jan 2019).
- Hop and Brew School podcast interview with Vinnie Cilurzo from Russian River Brewing, Jay Goodwin from The Rare Barrel and Charlie Johnson from the Ronin Fermentation Project on using fresh and aged hops in sour beer.
References
- ↑ "Hops". Wikipedia. Retrieved 06/10/2017.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 The Bitterness Intensity of Oxidized Hop Acids: Humulinones and Hulupones. Victor Alexander Algazzali for the degree of Master of Science in Food Science and Technology presented on August 8, 2014.
- ↑ HUMULUS LUPULUS L. (HOPS) – A VALUABLE SOURCE OF COMPOUNDS WITH BIOACTIVE EFFECTS FOR FUTURE THERAPIES. Jana Olšovská et al. 2016.
- ↑ Fundamentals of beer and hop chemistry. Denis De Keukeleire. 1999.
- ↑ 5.0 5.1 5.2 5.3 Influencing Factors on Hop Isomerization Beyond the Conventional Range. Nele Bastgen, Tobias Becher & Jean Titze. 2019. DOI: https://doi.org/10.1080/03610470.2019.1587734.
- ↑ 6.0 6.1 Schönberger and Kostelecky, 2012
- ↑ 7.0 7.1 7.2 Qualitative Determination of β‑Acids and Their Transformation Products in Beer and Hop Using HR/AM-LC-MS/MS. Martin Dušek, Jana Olšovská, Karel Krofta, Marie Jurková, and Alexandr Mikyška. 2014.
- ↑ 8.0 8.1 John Paul Maye. EBC 2017 Presentation. 2017.]
- ↑ CHANGES IN HOP ACIDS CONCENTRATIONS ON HEATING IN AQUEOUS SOLUTIONS AND UNHOPPED WORTS. H. O. Askew. 1964.
- ↑ 10.0 10.1 Isomerization and Degradation Kinetics of Hop (Humulus lupulus) Acids in a Model Wort-Boiling System. Mark G. Malowicki and Thomas H. Shellhammer. 2005.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Tracking IBU Through the Brewing Process: The Quest for Consistency. Aaron Justus. Director of R&D and Specialty Brewing, Ballast Point Brewing. MBAA TQ 2018; vol. 55, no.3. https://doi.org/10.1094/TQ-55-3-1205-01.
- ↑ Kinetic Modeling of Hop Acids during Wort Boiling. Yarong Huang, Johannes Tippmann, and Thomas Becker. 2013.
- ↑ Lars Marius Garshol. "Raw ale". Larsblog. 05/06/2015. Retrieved 12/17/2018.
- ↑ A Look at Isomerization Reduction Due to Altitude. John Palmer. MBAA TQ 2017 http://dx.doi.org/10.1094/TQ-54-3-0806-01.
- ↑ 15.0 15.1 15.2 15.3 15.4 Shellhammer, Vollmer, and Sharp. Oral presentation at the Craft Brewers Conference, 2015.
- ↑ 16.0 16.1 16.2 16.3 "Recent Advances in Controlling Hoppy Aroma in Beer." Daniel C. Sharp. OSU Brewing Science Presentation.
- ↑ 17.0 17.1 Comparison of 4-Mercapto-4-methylpentan-2-one Contents in Hop Cultivars from Different Growing Regions. Toru Kishimoto, Minoru Kobayashi, Nana Yako, Ayako Iida and Akira Wanikawa. 2008.
- ↑ "Geraniol". Wikipedia. Retrieved 01/09/2017.
- ↑ 19.0 19.1 Identification and Characteristics of New Volatile Thiols Derived from the Hop (Humulus luplus L.) Cultivar Nelson Sauvin. Kiyoshi Takoi, Marie Degueil, Svitlana Shinkaruk, Cécile Thibon, Katsuaki Maeda, Kazutoshi Ito, Bernard Bennetau, Denis Dubourdieu and Takatoshi Tominaga. 2009.
- ↑ 20.0 20.1 VOLATILE ORGANOSULPHUR COMPOUNDS IN HOPS AND HOP OILS: A REVIEW. T.L. Peppard. 1981.
- ↑ 21.0 21.1 21.2 3‑Sulfanyl-4-methylpentan-1-ol in Dry-Hopped Beers: First Evidence of Glutathione S‑Conjugates in Hop (Humulus lupulus L.). Marie-Lucie Kankolongo Cibaka, Laura Decourriere, Celso-JoséLorenzo-Alonso, Etienne Bodart, Raphael Robiette, and Sonia Collin. 2016.
- ↑ 22.0 22.1 The Language of Hops: How to Assess Hop Flavor in Hops and Beer. Georg Drexler, Elisabeth Wiesen, Mark Zunkel, Sebastian Hinz, Alicia Muñoz Insa, Victor Algazzali, Tim Kostelecky, and Christina Schönberger. 1. Joh. Barth & Sohn GmbH & Co. KG, Nuremberg, Germany. 2. John I. Haas Inc., Yakima, WA, U.S.A. MBAA Technical Quarterly. Vol. 54, no. 1. 2017. Pgs. 34–37. DOI: http://dx.doi.org/10.1094/TQ-54-1-0143-01.
- ↑ 23.0 23.1 J. S. Hough, B.Sc, Ph.D., G. A. Howard, M.Sc., Ph.D., and C. A. Slater, Ph.D. 1957.
- ↑ 24.0 24.1 SIGNIFICANCE OF THE USE OF HOPS IN REGARD TO THE BIOLOGICAL STABILITY OF BEER: I. REVIEW AND PRELIMINARY STUDIES. R. M. Macrae. 1964.
- ↑ 25.0 25.1 Fernandez and Simpson (1993)
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 Sakamoto and Konings (2003)
- ↑ 27.0 27.1 Heterogeneity between and within Strains of Lactobacillus brevis Exposed to Beer Compounds. Yu Zhao, Susanne Knøchel and Henrik Siegumfeldt. 2017. DOI: https://doi.org/10.3389/fmicb.2017.00239.
- ↑ 28.0 28.1 28.2 Schurr et al., (2015)
- ↑ The absolute configuration of the isohumulones and the humulinic acids. D.De Keukeleire, M.Verzele. 1971. https://doi.org/10.1016/S0040-4020(01)98199-2.
- ↑ 30.0 30.1 30.2 Behr and Vogel, (2010)
- ↑ R. Stevens, Ph.D., F.R.I.C., and D. Wright, Ph.D. 1961.
- ↑ Simpson and Fernandez, 1992
- ↑ 33.0 33.1 33.2 33.3 Biotransformations of hop-derived aroma compounds by Saccharomyces cerevisiae upon fermentation. Tatiana Praet, Filip Van Opstaele, Barbara Jaskula-Goiris, Guido Aerts, Luc De Cooman. 2012.
- ↑ "Biotransformation". Wikipedia. Retrieved 05/10/2019.
- ↑ "(Z)-rose oxide ". Good Scents Company. Retrieved 12/29/2016.
- ↑ "There are no flies on Emma Stoye". Emma Stoye. Education in Chemistry website. 06/01/2016. Retrieved 01/10/2017.
- ↑ "Citronellyl acetate". Perfumers Apprentice website. Retrieved 01/10/2017.
- ↑ The Contribution of Geraniol Metabolism to the Citrus Flavour of Beer: Synergy of Geraniol and β‐Citronellol Under Coexistence with Excess Linalool. Kiyoshi Takoi, Yutaka Itoga, Koichiro Koie, Takayuki Kosugi, Masayuki Shimase, Yuta Katayama, Yasuyuki Nakayama, Junji Watari. 2012. DOI: https://doi.org/10.1002/j.2050-0416.2010.tb00428.x.
- ↑ Screening of Geraniol-rich Flavor Hop and Interesting Behavior of beta-Citronellol During Fermentation under Various Hop-Addition Timings. Takoi, Kiyoshi & Itoga, Yutaka & Takayanagi, Junji & Kosugi, Takayuki & Shioi, Toru & Nakamura, Takeshi & Watari, Junji. 2014. DOI: 10.1094/ASBCJ-2014-0116-01.
- ↑ Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. King A1, Richard Dickinson J. 2000.
- ↑ Biotransformation of acyclic monoterpenoids by Debaryomyces sp., Kluyveromyces sp., and Pichia sp. strains of environmental origin. Ponzoni C, Gasparetti C, Goretti M, Turchetti B, Pagnoni UM, Cramarossa MR, Forti L, Buzzini P. 2008.
- ↑ "Influence of yeast strain on hop aroma development in dry hopped beers." Christina Schönberger, Elisabeth Wiesen, Benedikt Matsche, Barth Innovations Yves Gosselin, Stephan Meulemans, Fermentis. Presentation slides at 35th Congress EBC.
- ↑ "Optimizing hop aroma in beer dry hopped with Cascade utilizing glycosidic enzymes (presentation slides)." Kaylyn Kirkpatrick from New Belgium Brewing Co. Young Scientist Symposium, Chico, CA 2016.
- ↑ "Seeded and "Unseeded Hops - a Quality Comparison (presentation slides)." Martin Zarnkow. EBC 2015.
- ↑ Octanol. The Good Scents Company. Retrieved 03/31/2017.
- ↑ The effect of hopping regime, cultivar and β-glucosidase activity on monoterpene alcohol concentrations in wort and beer. Daniel C. Sharp, Jan Steensels, Thomas H. Shellhammer. 2017. DOI: 10.1021/jf2042517.
- ↑ Eric G. Dennis, Robert A. Keyzers, Curtis M. Kalua, Suzanne M. Maffei, Emily L. Nicholson, and Paul K. Boss. 2012.
- ↑ Microbial acidification, alcoholization, and aroma production during spontaneous lambic beer production. Jonas De Roos and Luc De Vuyst. 2018. DOI: 10.1002/jsfa.9291.
- ↑ Conversation with Ron Smith and Aaron Barker on historical storage of hops. 02/07/2016.
- ↑ Dave Janssen. "Hops in spontaneous fermentation". Hors Catégorie Brewing blog. 04/28/2016. Retrieved 04/09/2018.
- ↑ "Home for Our Aged Hops". Jester King's blog. Retrieved 11/18/2016.
- ↑ 52.0 52.1 52.2 52.3 Aging of Hops and Their Contribution to Beer Flavor. Lam et al. 1986.
- ↑ 53.0 53.1 53.2 53.3 Humulinone Formation in Hops and Hop Pellets and Its Implications for Dry Hopped Beers. John Paul Maye, Robert Smith, and Jeremy Leker. 2016.
- ↑ 54.0 54.1 54.2 54.3 54.4 54.5 54.6 54.7 Assessment of changes in hop resins and polyphenols during long-term storage. Alexandr Mikyška and Karel Krofta. 2012.
- ↑ 55.0 55.1 Brewing Science and Practice. Dennis E. Briggs, Chris A. Boulton, Peter A. Brookes, Roger Stevens. 2004.
- ↑ 56.0 56.1 56.2 56.3 Stability of Hop Beta Acids and Their Decomposition Products during Natural Ageing. K. Krofta, S. Vrabcová, A. Mikyska, M. Jurková, T. Cajka , J. Hajslová. 2013.
- ↑ The effect of hop beta acids oxidation products on beer bitterness. Karel Krofta, Světlana VRABCOVÁ, Alexandr Mikyška, and Marie JURKOVÁ. 2013.
- ↑ Structure determination and sensory evaluation of novel bitter compounds formed from β-acids of hop (Humulus lupulus L.) upon wort boiling. Gesa Haseleu, Daniel Intelmann, Thomas Hofmann. 2009.
- ↑ 59.0 59.1 Dr. Patricia Aron. "Bitterness and the IBU: What’s It All About?" HomebrewCon 2017 Presentation. ~34 mins in. Retrieved 09/05/2017.
- ↑ Adam Kielich. "One Gallon Spontaneous Fermentation Beer Batch 5 Recipe and Brewday". Brain Sparging on Brewing. 11/16/2019.
- ↑ Goiris et al., 2002
- ↑ Beta-ionone. Good Scents Company. Retrieved 11/22/2016.
- ↑ 63.0 63.1 63.2 Odorants comprising hop aroma of beer: hop-derived odorants increased in the beer hopped with aged hops. Toru Kishimoto, Katsunori Kono and Kenkichi Aoki. 2007.
- ↑ Aroma Properties of Lager Beer Dry-Hopped with Oxidized Hops. Daniel M. Vollmer, Victor Algazzali, and Thomas H. Shellhammer. 2017.
- ↑ Comparison of the Odor-Active Compounds in Unhopped Beer and Beers Hopped with Different Hop Varieties. Toru Kishimoto, Akira Wanikawa, Katsunori Kono, and Kazunori Shibata. 2006.
- ↑ Contribution of Hop Bitter Substances to Beer Staling Mechanisms. Williams and Wagner. 1979.
- ↑ Aging characteristics of different beer types. Bart Vanderhaegen, Filip Delvaux, Luk Daenen, Hubert Verachtert, Freddy R.Delvaux. 2007. DOI: https://doi.org/10.1016/j.foodchem.2006.07.062.
- ↑ 3-methyl-2-butene-1-thiol. Aroxa website. Retrieved 11/22/2016.
- ↑ Private correspondence with Dustin Carver by Farmhouse Brewing Supply. 03/22/2016.
- ↑ James Howat. Milk The Funk Facebook group thread about debittered leaf cryohops. 07/04/2018.
- ↑ MTF thread that reported an MBAA presentation by Brett Porter from Goose Island. 07/30/2016.
- ↑ "Commercial Brettanomyces, Lactobacillus, and Pediococcus Descriptions; Commercial Yeast Laboratories." The Mad Fermentationist blog. Michael Tonsmeire. Retrieved 12/12/2016.
- ↑ "Classification of Brewed Products as “Beer” Under the Internal Revenue Code of 1986 and as “Malt Beverages” Under the Federal Alcohol Administration Act". TTB Ruling 2008, Number 2008-3. 07/07/2008. Retrieved 12/12/2016.
- ↑ MTF thread with John Joyce and Joseph Kearns on TTB vs FDA approval for beer/malt beverages without hops. 12/13/2016.
- ↑ "Putting Some Numbers on First Wort and Mash Hop additions." David Curtis. 2014 National Homebrewers Conference presentation slides. Retrieved 12/12/2016.
- ↑ "Raw ale". Larsblog. Lars Marius Garshol. 05/06/2015. Retrieved 12/12/2016.
- ↑ A Study of Factors Affecting the Extraction of Flavor When Dry Hopping Beer (master thesis). Peter Harold Wolfe. 2012.
- ↑ Nate Walter and Dan Pixley. Milk The Funk Facebook group. 05/21/2017.
- ↑ Janish, Scott. "Zero Hot-Side Hopped NEIPA | HPLC Testing for Sensory Bitterness". ScottJanish.com. Retrieved 03/09/2017.
- ↑ Carloine Whalen Taggart. Milk The Funk Facebook group. 07/06/2017.
- ↑ 81.0 81.1 81.2 81.3 The Freshening Power of Centennial Hops. Jacob A. Kirkendall, Carter A. Mitchell & Lucas R. Chadwick. 2018. DOI: https://doi.org/10.1080/03610470.2018.1469081.
- ↑ 82.0 82.1 Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). Kaylyn R. Kirkpatrick and Thomas H. Shellhammer. 2018. DOI: DOI: 10.1021/acs.jafc.8b03563.
- ↑ STEVE 'HENDO' HENDERSON. How “Dry Hop Creep” Causes Diacetyl In Beer and How Brewers Can Minimise The Risk. Rockstar Brewer Academy website. 09/03/2018. Retrieved 10/05/2018.
- ↑ Brad Smith. BeerSmith blog. 03/13/2019. Retrieved 07/23/2019.
- ↑ Jean van Roy on Basic Brewing Radio
- ↑ D. Janssen personal communication with Jean Van Roy, 9-Nov-2013
- ↑ 87.0 87.1 Drie Fonteinen on Belgian Smaak
- ↑ Averie Swanson. "Sour Power! A Pro Brewer Spontaneous Fermentation Roundtable". HomebrewCon seminar. 2018.
- ↑ Video of Cantillon wort reaching a boil from Bill on Lambic.info
- ↑ Conversation with Dave Janssen on MTF. 02/24/2017.
- ↑ "Brewing Lambic", section "Hopping". Lambic.info website. Retrieved 02/24/2017.
- ↑ Andrew Holzhauer. Milk The Funk Facebook group on how much aged hops to use. 06/13/2019.
- ↑ Caleb Buck. Milk the Funk Facebook group thread on Caleb's aged hop experiment. 10/01/2018.
- ↑ 94.0 94.1 94.2 94.3 94.4 Dave Janssen's discussion of hopping in spontaneous fermentation
- ↑ 95.0 95.1 Dave Janssen's discussion of hopping grisettes
- ↑ Howat, James. Facebook live video stream. 12/23/2016. ~5:30 minutes in.